A Comparative Study of Ferulic Acid on Different Monosaccharide-Mediated Protein Glycation and Oxidative Damage in Bovine Serum Albumin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Ferulic Acid on Advanced Glycation End-Products (AGEs) and Amadori Products
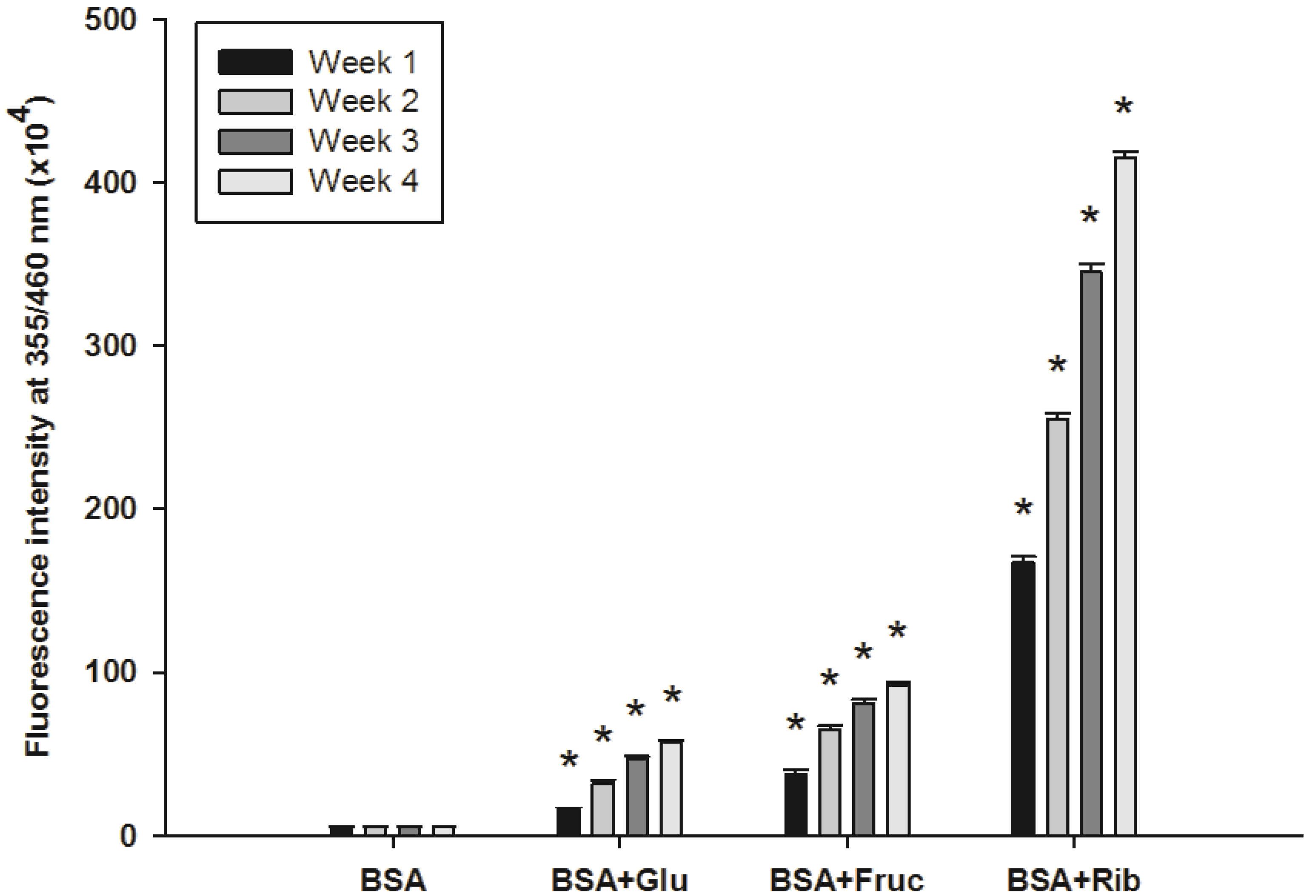


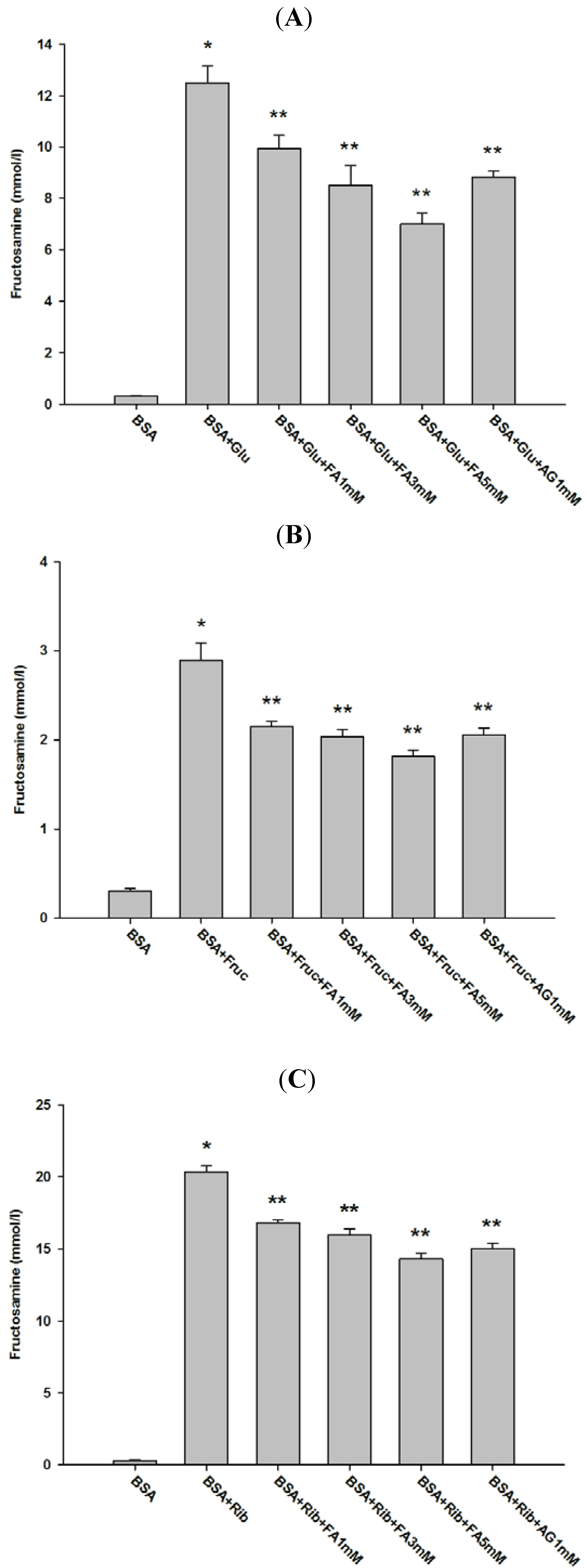
2.2. Effect of Ferulic Acid on Protein Aggregation

2.3. Effect of Ferulic Acid on Protein Thiol Group and Protein Carbonyl Content
| Experimental groups | Protein thiol group | Protein carbonyl content |
|---|---|---|
| (nmol/mg protein) | (nmol/mg protein) | |
| BSA | 0.92 ± 0.03 | 0.26 ± 0.04 |
| BSA+Glu | 0.75 ± 0.02 * | 2.03 ± 0.06 * |
| BSA+Glu+FA 1 mM | 0.80 ± 0.02 | 1.26 ± 0.04 ** |
| BSA+Glu+FA 3 mM | 0.83 ± 0.02 | 1.07 ± 0.03 ** |
| BSA+Glu+FA 5 mM | 0.85 ± 0.03 | 0.89 ± 0.04 ** |
| BSA+Glu+AG 1 mM | 0.88 ± 0.02 ** | 1.16 ± 0.06 ** |
| BSA+Fruc | 0.66 ± 0.03 * | 2.98 ± 0.08 * |
| BSA+Fruc+FA 1 mM | 0.70 ± 0.03 | 1.88 ± 0.04 ** |
| BSA+Fruc+FA 3 mM | 0.74 ± 0.01 | 1.68 ± 0.06 ** |
| BSA+Fruc+FA 5 mM | 0.79 ± 0.02 | 1.41 ± 0.03 ** |
| BSA+Fruc+AG 1 mM | 0.85 ± 0.04 ** | 1.77 ± 0.06 ** |
| BSA+Rib | 0.50 ± 0.01 * | 6.97 ± 0.09 * |
| BSA+Rib+FA 1 mM | 0.61 ± 0.02 | 5.19 ± 0.09 ** |
| BSA+Rib+FA 3 mM | 0.66 ± 0.05 ** | 4.93 ± 0.07 ** |
| BSA+Rib+FA 5 mM | 0.70 ± 0.02 ** | 4.64 ± 0.06 ** |
| BSA+Rib+AG 1 mM | 0.83 ± 0.02 ** | 4.79 ± 0.06 ** |
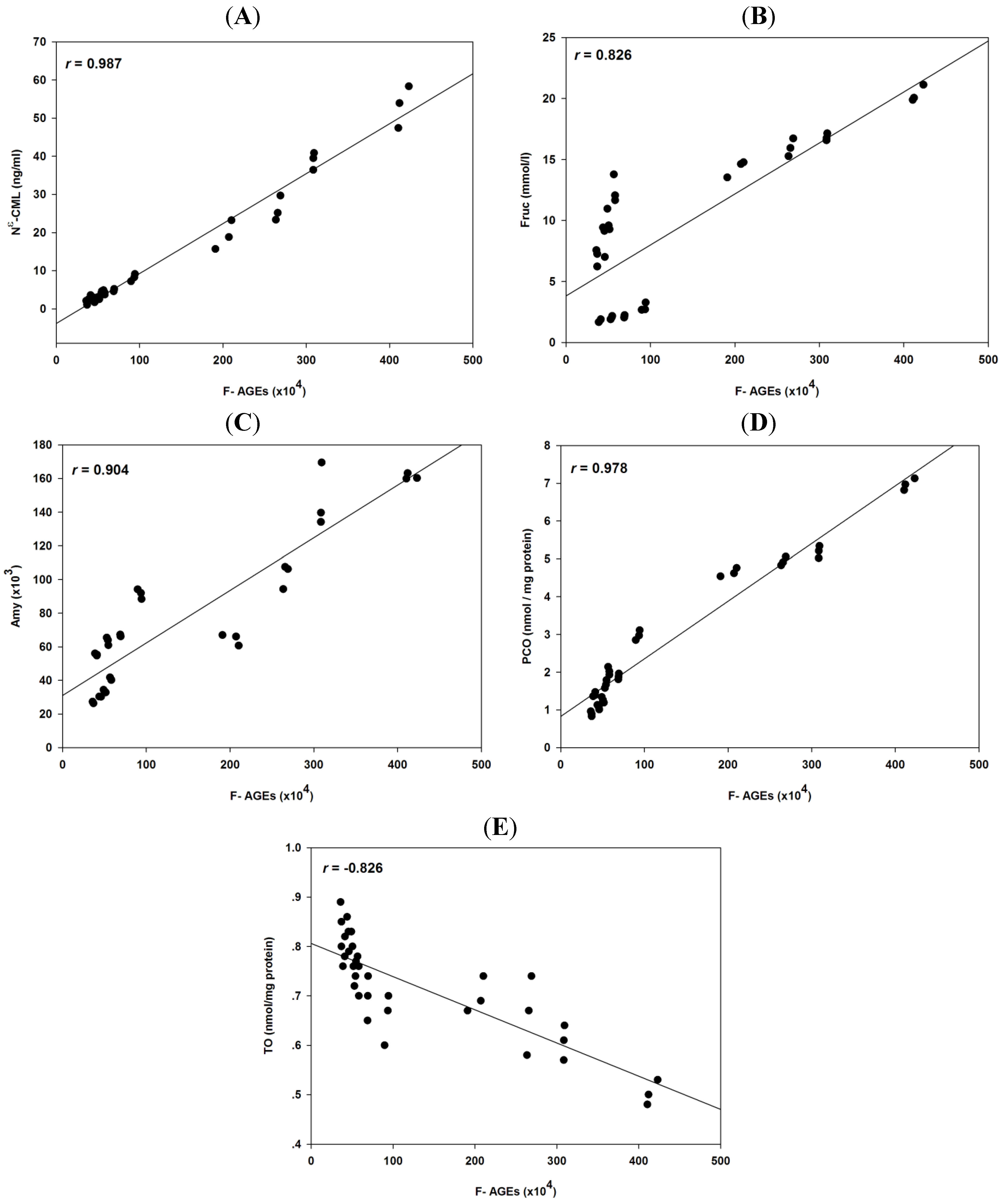
2.4. Correlation Coefficients between Glycation and Protein Oxidation
| F-AGEs | Nε-CML | Frc | Amy | PCO | TO | |
|---|---|---|---|---|---|---|
| F-AGEs | - | 0.987 * | 0.826 * | 0.904 * | 0.978 * | −0.826 * |
| Nε-CML | - | - | 0.806 * | 0.919 * | 0.956 * | −0.806 * |
| Frc | - | - | - | 0.572 * | 0.787 * | −0.512 * |
| Amy | - | - | - | - | 0.896 * | −0.876 * |
| PCO | - | - | - | - | - | −0.845 * |
| TO | - | - | - | - | - | - |
3. Experimental
3.1. Chemicals
3.2. In Vitro Glycation of Bovine Serum Albumin (BSA) Induced by Monosaccharides
3.3. Determination of Advanced Glycation End-Products (AGEs)
3.4. Determination of Fructosamine (Amadori Products)
3.5. Determination of Protein Aggregation
3.6. Determination of Protein Thiol Group
3.7. Determination of Protein Carbonyl Content
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Baynes, J.W.; Watkins, N.G.; Fisher, C.I.; Hull, C.J.; Patrick, J.S.; Ahmed, M.U.; Dunn, J.A.; Thorpe, S.R. The Amadori product on protein: Structure and reactions. Prog. Clin. Biol. 1989, 304, 43–67. [Google Scholar]
- Booth, A.A.; Khalifah, R.G.; Todd, P.; Hudson, B.G. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J. Biol. Chem. 1997, 272, 5430–5437. [Google Scholar]
- Cohen, G.; Riahi, Y.; Alpert, E.; Gruzman, A.; Sasson, S. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch. Physiol. Biochem. 2007, 113, 259–267. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, S.M.; Lin, J.A.; Yen, G.C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011, 2, 224–234. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Stern, D. Atherosclerosis and diabetes: The RAGE connection. Curr. Atheroscler. Rep. 2000, 2, 430–436. [Google Scholar] [CrossRef]
- Vitek, M.P.; Bhattacharya, K.; Glendening, J.M.; Stopa, E.; Vlassara, H.; Bucala, R.; Manogue, K.; Ceremi, A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 4766–4770. [Google Scholar] [CrossRef]
- Ledesma-Osuna, A.I.; Ramos-Clamont, G.; Vazquez-Moreno, L. Characterization of bovine serum albumin glycated with glucose, galactose and lactose. Acta Biochim. Pol. 2008, 55, 491–497. [Google Scholar]
- Wei, Y.; Chen, L.; Chen, J.; Ge, L.; He, R.Q. Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009, 10, 10. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Moonsan, P.; Yibchok-Anun, S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 7838–7844. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. J. Enzyme Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y. Protein glycation inhibitory activity of wheat bran feruloyl oligosaccharides. Food Chem. 2009, 112, 350–353. [Google Scholar] [CrossRef]
- Srey, C.; Hull, G.L.; Connolly, L.; Elliott, C.T.; del Castillo, M.D.; Ames, J.M. Effect of inhibitor compounds on Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL) formation in model foods. J. Agric. Food Chem. 2010, 58, 12036–12041. [Google Scholar] [CrossRef]
- Srey, C.; Haughey, S.A.; Connolly, L.; del Castillo, M.D.; Ames, J.M.; Elliott, C.T. Immunochemical and mass spectrometric analysis of Nε-(carboxymethyl)lysine content of AGE-BSA systems prepared with and without selected antiglycation agents. J. Agric. Food Chem. 2010, 58, 11955–11961. [Google Scholar] [CrossRef] [Green Version]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef]
- Monnier, V.M. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J. Gerontol. 1990, 45, B105–B111. [Google Scholar] [CrossRef]
- Silvan, J.M.; Assar, S.H.; Srey, C.; Dolores del Castillo, M.; Ames, J.M. Control of the Maillard reaction by ferulic acid. Food Chem. 2011, 128, 208–213. [Google Scholar] [CrossRef]
- Hinton, D.; Ames, J. Site specificity of glycation and carboxymethylation of bovine serum albumin by fructose. Amino Acids 2006, 30, 425–434. [Google Scholar] [CrossRef]
- Vukasovi, D.; Jureti, D. Enzyme activity and AGE formation in a model of AST glycoxidation by D-fructose in vitro. Acta Pharm. 2005, 55, 107–114. [Google Scholar]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-anun, S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.; Schulze, J.; Souza, M.F. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, H.W.; Lin, J.A.; Huang, S.M.; Yen, G.C. The proglycation effect of caffeic acid leads to the elevation of oxidative stress and inflammation in monocytes, macrophages and vascular endothelial cells. J. Nutr. Biochem. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Meeprom, A.; Sompong, W.; Chan, C.B.; Adisakwattana, S. Isoferulic Acid, a new anti-glycation agent, inhibits fructose-and glucose-mediated protein glycation in vitro. Molecules 2013, 18, 6439–6454. [Google Scholar] [CrossRef]
- Suarez, G.; Rajaram, R.; Oronsky, A.; Gawinowicz, M. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J. Biol. Chem. 1989, 264, 3674–3679. [Google Scholar]
- Ahmed, N.; Furth, A.J. Failure of common glycation assays to detect glycation by fructose. Clin. Chem. 1992, 38, 1301–1303. [Google Scholar]
- Foiles, P.G.; Founds, H.W.; Vasan, S. Therapeutic potential of AGE inhibitors and breakers of AGE protein cross-links. Expert Opin. Invest. Drugs. 2001, 10, 1977–1987. [Google Scholar] [CrossRef]
- Bouma, B.; Kroon-Batenburg, L.M.; Wu, Y.P.; Brunjes, B.; Posthuma, G.; Kranenburg, O.; de Groot, P.G.; Voest, E.E.; Gebbink, M.F. Glycation induces formation of amyloid cross-beta structure in albumin. J. Biol. Chem. 2003, 278, 41810–41819. [Google Scholar] [CrossRef]
- Luthra, M.; Balasubramanian, D. Nonenzymatic glycation alters protein structure and stability. A study of two eye lens crystallins. J. Biol. Chem. 1993, 268, 18119–18127. [Google Scholar]
- Tokuyama, T.; Yagui, K.; Yamaguchi, T.; Huang, C.I.; Kuramoto, N.; Shimada, F.; Miyazaki, J.; Horie, H.; Saito, Y.; Makino, H.; et al. Expression of human islet amyloid polypeptide/amylin impairs insulin secretion in mouse pancreatic beta cells. Metabolism 1997, 46, 1044–1051. [Google Scholar] [CrossRef]
- Marzban, L.; Verchere, C.B. The role of islet amyloid polypeptide in type 2 diabetes. Can. J. Diabetes 2004, 28, 39–47. [Google Scholar]
- Zeng, J.; Davies, M.J. Protein and low molecular mass thiols as targets and inhibitors of glycation reactions. Chem. Res. Toxicol. 2006, 19, 1668–1676. [Google Scholar] [CrossRef]
- Acimovic, J.M.; Stanimirovic, B.D.; Mandic, L.M. The role of the thiol group in protein modification with methylglyoxal. J. Serb. Chem. Soc. 2009, 74, 867–883. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Carubelli, R.; Schneider, J.E.; Pye, Q.N.; Floyd, R.A. Cytotoxic effects of autoxidative glycation. Free Radic. Biol. Med. 1995, 18, 265–269. [Google Scholar] [CrossRef]
- Smith, P.R.; Thornalley, P.J. Mechanism of the degradation of non-enzymatically glycated proteins under physiological conditions. Eur. J. Biochem. 1992, 210, 729–739. [Google Scholar] [CrossRef]
- Nagai, R.; Ikeda, K.; Higashi, T.; Sano, H.; Jinnouchi, Y.; Araki, T.; Horiuchi, S. Hydroxyl Radical Mediates Nϵ-(Carboxymethyl) lysine Formation from Amadori Product. Biochem. Biophys. Res. Commun. 1997, 234, 167–172. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2001, 22, 2711–2717. [Google Scholar]
- Peyroux, J.; Sternberg, M. Advanced glycation endproducts (AGEs): Pharmacological inhibition in diabetes. Pathol. Biol. 2006, 54, 405–419. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Jiphimai, P.; Prutanopajai, P.; Chanathong, B.; Sapwarobol, S.; Ariyapitipan, T. Evaluation of α-glucosidase, α-amylase and protein glycation inhibitory activities of edible plants. Int. J. Food Sci. Nutr. 2010, 61, 295–305. [Google Scholar] [CrossRef]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar] [CrossRef]
- Le Vine, H. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999, 309, 274–284. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E.; Osawa, T. Protein-bound acrolein: Potential markers for oxidative stress (covalent modification of protein/antibody/atherosclerosis). Proc. Natl. Acad. Sci. USA 1998, 95, 4882–4887. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sompong, W.; Meeprom, A.; Cheng, H.; Adisakwattana, S. A Comparative Study of Ferulic Acid on Different Monosaccharide-Mediated Protein Glycation and Oxidative Damage in Bovine Serum Albumin. Molecules 2013, 18, 13886-13903. https://doi.org/10.3390/molecules181113886
Sompong W, Meeprom A, Cheng H, Adisakwattana S. A Comparative Study of Ferulic Acid on Different Monosaccharide-Mediated Protein Glycation and Oxidative Damage in Bovine Serum Albumin. Molecules. 2013; 18(11):13886-13903. https://doi.org/10.3390/molecules181113886
Chicago/Turabian StyleSompong, Weerachat, Aramsri Meeprom, Henrique Cheng, and Sirichai Adisakwattana. 2013. "A Comparative Study of Ferulic Acid on Different Monosaccharide-Mediated Protein Glycation and Oxidative Damage in Bovine Serum Albumin" Molecules 18, no. 11: 13886-13903. https://doi.org/10.3390/molecules181113886






