One Lignanoid Compound and Four Triterpenoid Compounds with Anti-Inflammatory Activity from the Leaves of Elaeagnus oldhamii Maxim.
Abstract
:1. Introduction
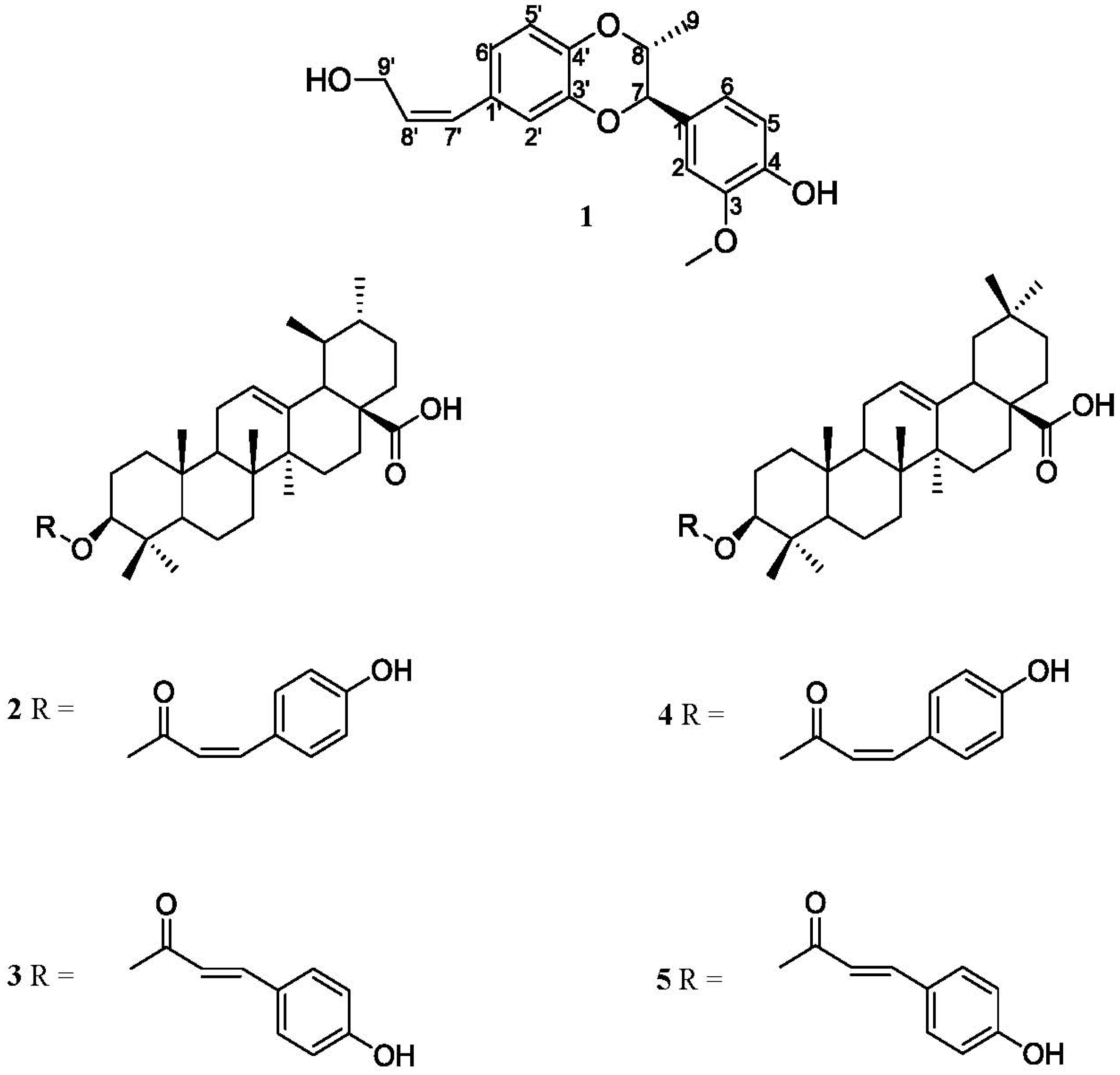
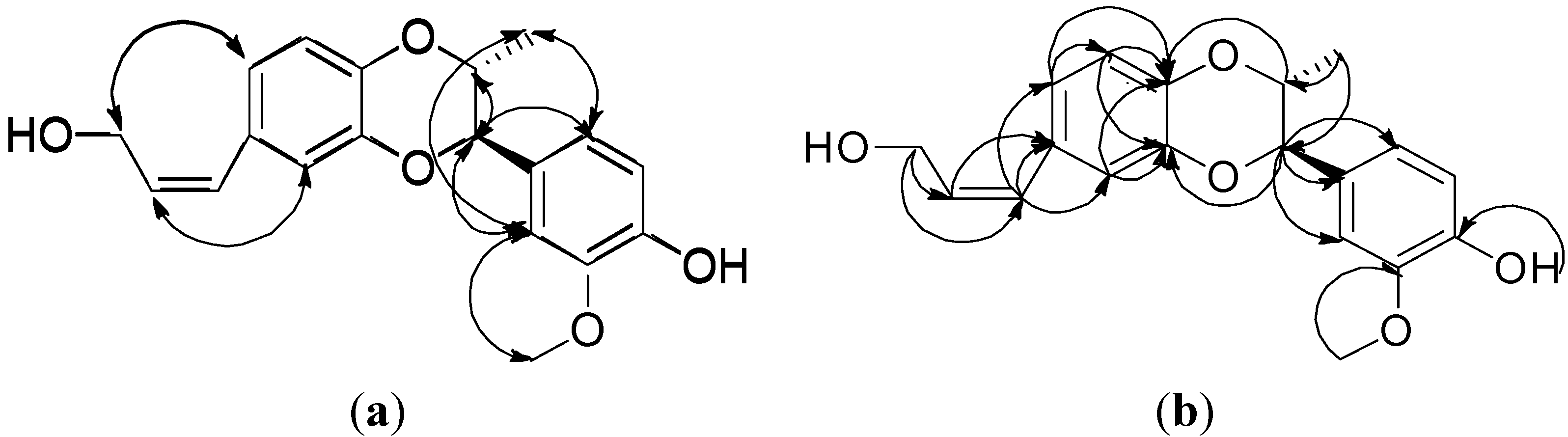
2. Results and Discussion
| Position | δH | δC |
|---|---|---|
| 1 | - | 129.39 |
| 2 | 7.06 (d, J = 1.6, 1H) | 111.30 |
| 3 | - | 148.50 |
| 4 | - | 147.57 |
| 5 | 6.87 (d, J = 8.2, 1H) | 117.92 |
| 6 | 6.92 (dd, J = 8.2, 1.6, 1H) | 120.30 |
| 7 | 5.18 (d, J = 2.6, 1H) | 78.13 |
| 8 | 4.60 (qd, J = 6.6, 2.6, 1H) | 73.81 |
| 9 | 1.08 (d, J = 6.6, 3H) | 13.71 |
| Phenyl-OMe | 3.83 (s, 3H) | 56.48 |
| Phenyl-OH | 7.69 (s, H) | - |
| 1′ | - | 132.77 |
| 2′ | 6.87 (d, J = 1.8, 1H) | 118.25 |
| 3′ | - | 143.94 |
| 4′ | - | 142.57 |
| 5′ | 6.86 (d, J = 8.2, 1H) | 115.92 |
| 6′ | 6.79 (dd, J = 8.2, 1.8, 1H) | 123.45 |
| 7′ | 6.38 (d, J = 11.8,1H) | 129.64 |
| 8′ | 5.76 (m, 1H) | 131.54 |
| 9′ | 4.38 (d, J = 5.0, 2H) | 59.83 |
| OH | 3.89 (br s, H) | - |
| Compound | Dose (μg/mL) | Cell Viability (% of Control) | No Level | NO Inhibition (% of Control) | IC50 (μg/mL) |
|---|---|---|---|---|---|
| Control | (−) | 98.2 ± 4.4 | −0.1 ± 0.3 | ± | |
| LPS | (+) | 100.5 ± 4.0 | 30.3 ± 2.2 ### | ± | |
| 1 | 2.5 | 100.6 ± 5.9 | 17.6 ± 0.5 *** | 42.0 ± 1.6 | |
| 5 | 97.6 ± 4.3 | 17.5 ± 08 *** | 42.3 ± 2.6 | ||
| 10 | 90.7 ± 3.3 | 15.2 ± 0.4 *** | 49.7 ± 1.4 | 10.3 ± 0.4 | |
| 20 | 88.8 ± 2.6 | 12.0 ± 0.4 *** | 60.4 ± 1.3 | ||
| 2 | 2.5 | 87.2 ± 2.0 | 19.7 ± 1.4 ** | 35.1 ± 4.6 | |
| 5 | 77.9 ± 5.0 | (−) | (−) | ||
| 10 | 43.4 ± 1.6 | (−) | (−) | ||
| 20 | 28.0 ± 2.9 | (−) | (−) | ||
| 3 | 2.5 | 87.6 ± 5.6 | 23.5 ± 1.4 ** | 22.5 ± 4.6 | |
| 5 | 86.6 ± 4.2 | 21.0 ± 0.7 *** | 30.7 ± 2.2 | ||
| 10 | 74.1 ± 3.9 | (−) | (−) | ||
| 20 | 49.7 ± 8.2 | (−) | (−) | ||
| 4 | 2.5 | 90.8 ± 5.4 | 23.0 ± 2.8 *** | 24.2 ± 9.1 | |
| 5 | 87.4 ± 3.1 | 20.8 ± 1.8 *** | 31.3 ± 5.8 | ||
| 10 | 70.3 ± 3.5 | (−) | (−) | ||
| 20 | 47.6 ± 9.7 | (−) | (−) | ||
| 5 | 2.5 | 93.0 ± 8.6 | 20.5 ± 2.6 *** | 32.3 ± 8.6 | |
| 5 | 88.8 ± 5.7 | 19.5 ± 1.8 *** | 35.6 ± 6.1 | ||
| 10 | 88.5 ± 7.4 | 18.4 ± 0.8 *** | 39.3 ± 2.6 | >20 | |
| 20 | 86.5 ± 6.1 | 17.2 ± 0.9 *** | 43.0 ± 3.1 |
3. Experimental
3.1. General
3.2. Collection, Extraction and Isolation
3.3. Isoamericanol B (1)
3.4. Chemicals
3.5. Cell Culture
3.6. Cell Viability
3.7. Measurement of Nitric Oxide/Nitrite
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Huang, T.S. Elaeagnaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1998; Volume 3, pp. 753–759. [Google Scholar]
- Ahmad, S.D.; Sabir, M.S.; Juma, M.; Asad, H.S. Morphological and biochemical variations in Elaeagnus umbellata Thunb. from mountains of Pakistan. Acta Bot. Croat. 2005, 64, 121–128. [Google Scholar]
- Yuebin, G.; Liu, J.; Su, D. In vivo evaluation of the anti-asthmatic, antitussive and expectorant activities of extract and fractions from Elaeagnus pungens leaf. J. Ethnopharmacol. 2009, 126, 538–542. [Google Scholar]
- Ahmadiani, A.; Hosseiny, J.; Semnanian, S.; Javan, M.; Saeedi, F.; Kamalinejad, M.; Saremi, S. Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J. Ethnopharmacol. 2000, 72, 287–292. [Google Scholar] [CrossRef]
- Mehrabani Natanzi, M.; Pasalar, P.; Kamalinejad, M.; Dehpour, A.R.; Tavangar, S.M.; Sharifi, R.; Ghanadian, N.; Rahimi-Balaei, M.; Gerayesh-Nejad, S. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Med. Iran 2012, 50, 589–596. [Google Scholar]
- Lee, Y.S.; Chang, Z.Q.; Oh, B.C.; Park, S.C.; Shin, S.R.; Kim, N.W. Antioxidant activity, anti-inflammatory activity, and whitening effects of extracts of Elaeagnus multiflora Thunb. J. Med. Food 2007, 10, 126–133. [Google Scholar]
- Fu, Y.C.; Wang, X.J. Advances on chemical constituents and pharmacological activities from plants of Elaeagnus L. Qilu Pharmaceutical Affairs 2007, 26, 232–233. [Google Scholar]
- Lou, F.M.; Yang, J.; Bai, Z.C.; Wu, B.F. Studies on chemical constituents in rhizome of Elaeagnus bockii. Zhongguo Zhong Yao Za Zhi 2006, 31, 988–989. [Google Scholar]
- Ayaz, M.; Riaz, M.; Malik, A.; Ahmad, E.; Fatima, I. Elaeagnoside, chymotrypsin inhibiting steroidal glucoside from Elaeagnus orientalis. Nat. Prod. Res. 2009, 23, 409–414. [Google Scholar] [CrossRef]
- Ge, Y.B.; Li, M.S.; Mei, Z.N.; Yang, G.Z. Two new flavonol glycosides from the leaves of Elaeagnus pungens. J. Asian Nat. Prod. Res. 2013. [Google Scholar] [CrossRef]
- Wu, Y.B.; Gu, Y.; Ouyang, M.A. Water-soluble constituents from the bark of Elaeagnus pungens and their cytotoxic activities. J. Asian Nat. Prod. Res. 2010, 12, 278–285. [Google Scholar] [CrossRef]
- Liao, C.R.; Chang, Y.S.; Peng, W.H.; Lai, S.C.; Ho, Y.L. Analgesic and anti-inflammatory activities of the methanol extract of Elaeagnus oldhamii Maxim. in mice. Am. J. Chin. Med. 2012, 40, 581–597. [Google Scholar] [CrossRef]
- Song, W.W.; Li, B.; Liu, J.K. A new lignan from Elaeagnus lanceolata (Elaeagnaceae). Yunnan Zhiwu Yanjiu 2010, 32, 455–462. [Google Scholar]
- Ishikawa, T.; Seki, M.; Nishigaya, K.; Miura, Y.; Seki, H.; Chen, I.S.; Ishii, H. Studies on the chemical constituents of Xanthoxylum nitidum (Roxb.) D. C. (Fagara nitida Roxb.). III. The chemical constituents of the wood. Chem. Pharm. Bull. 1995, 43, 2014–2018. [Google Scholar] [CrossRef]
- Takahashi, H.; Luchi, M.; Fujita, Y.; Minami, H.; Fukuyama, Y. Coumaroyl triterpenes from Casuarina equisetifolia. Phytochemistry 1999, 51, 543–550. [Google Scholar]
- He, X.; Liu, R.H. Cranberryphytochemicals: Isolation, structureelucidation, and their antiproliferative and antioxidant activities. J. Agric. Food Chem. 2006, 54, 7069–7074. [Google Scholar] [CrossRef]
- Katai, M.; Terai, T.; Meguri, H. Triterpenoids of the barks of Pieris japonica D. Don (Japanese name: Asebi). II. 13C nuclear magnetic resonance of the gamma-lactones of ursane- and oleanane-type triterpenes. Chem. Pharm. Bull. 1983, 31, 1567–1571. [Google Scholar] [CrossRef]
- Pan, C.; Kim, E.S.; Jung, S.H.; Nho, C.W.; Lee, J.K. TectorigenininhibitsIFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch. Pharm. Res. 2008, 31, 1447–1456. [Google Scholar]
- Lee, Y.J.; Eun, J.R. Cilostazoldecreasesethanol-mediatedTNF alphaexpression in RAW264.7 murine macrophage and in liver from binge drinking mice. Korean J. Physiol. Pharmacol. 2012, 16, 131–138. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liao, C.-R.; Ho, Y.-L.; Huang, G.-J.; Yang, C.S.; Chao, C.-Y.; Chang, Y.-S.; Kuo, Y.-H. One Lignanoid Compound and Four Triterpenoid Compounds with Anti-Inflammatory Activity from the Leaves of Elaeagnus oldhamii Maxim. Molecules 2013, 18, 13218-13227. https://doi.org/10.3390/molecules181113218
Liao C-R, Ho Y-L, Huang G-J, Yang CS, Chao C-Y, Chang Y-S, Kuo Y-H. One Lignanoid Compound and Four Triterpenoid Compounds with Anti-Inflammatory Activity from the Leaves of Elaeagnus oldhamii Maxim. Molecules. 2013; 18(11):13218-13227. https://doi.org/10.3390/molecules181113218
Chicago/Turabian StyleLiao, Chi-Ren, Yu-Ling Ho, Guan-Jhong Huang, Chang Syun Yang, Che-Yi Chao, Yuan-Shiun Chang, and Yueh-Hsiung Kuo. 2013. "One Lignanoid Compound and Four Triterpenoid Compounds with Anti-Inflammatory Activity from the Leaves of Elaeagnus oldhamii Maxim." Molecules 18, no. 11: 13218-13227. https://doi.org/10.3390/molecules181113218





