Genistein Enhances the Radiosensitivity of Breast Cancer Cells via G2/M Cell Cycle Arrest and Apoptosis
Abstract
:1. Introduction

2. Results and Discussion
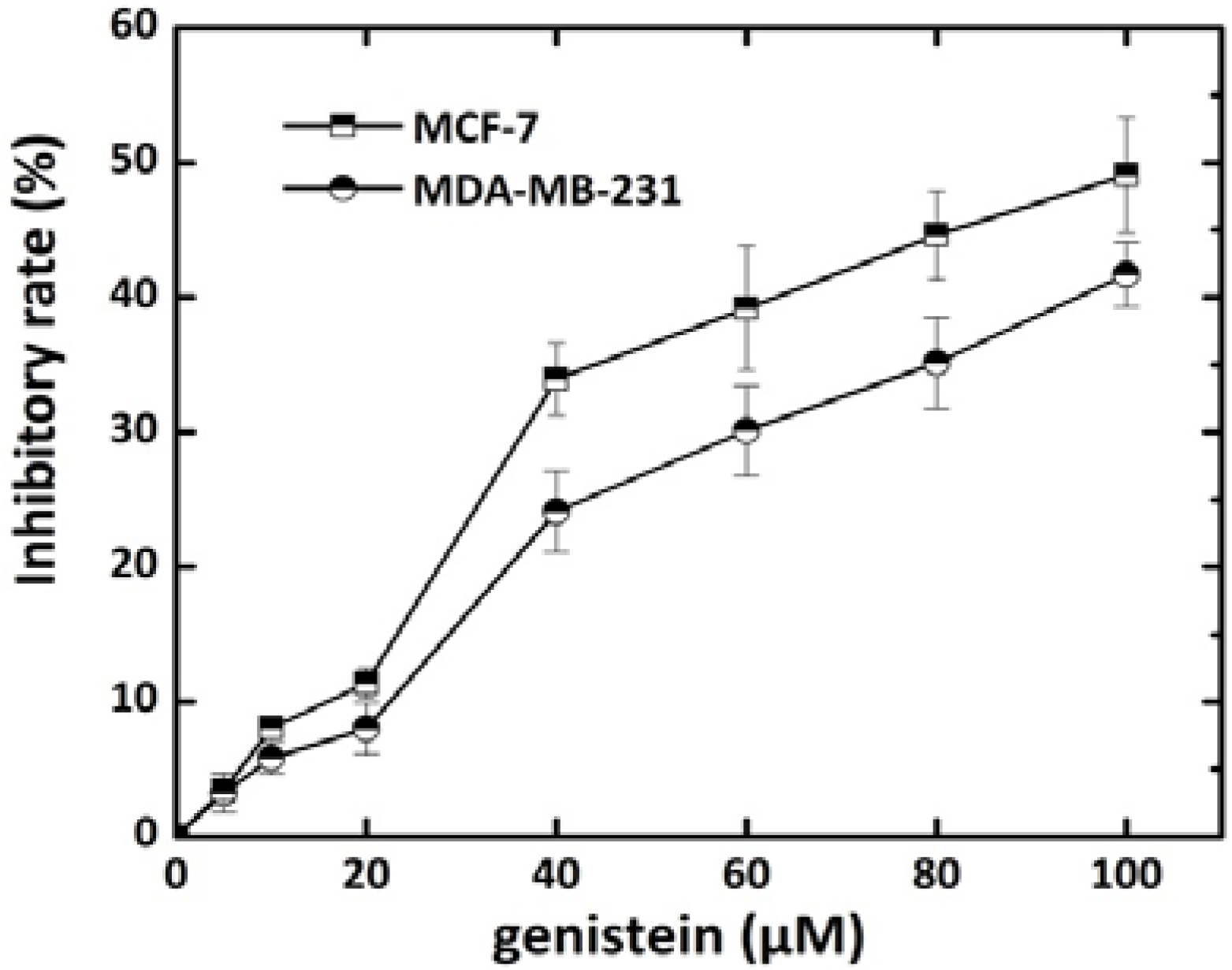
2.1. Effect of Genistein on Breast Cancer Cell Growth
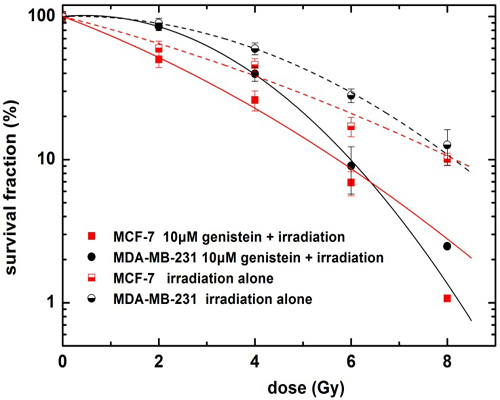
2.2. Genistein Pretreatment Followed by Irradiation with X-rays Reduced the Colony Forming Ability

2.3. Genistein Pretreatment Followed by Irradiation with X-rays Increased DNA Damage

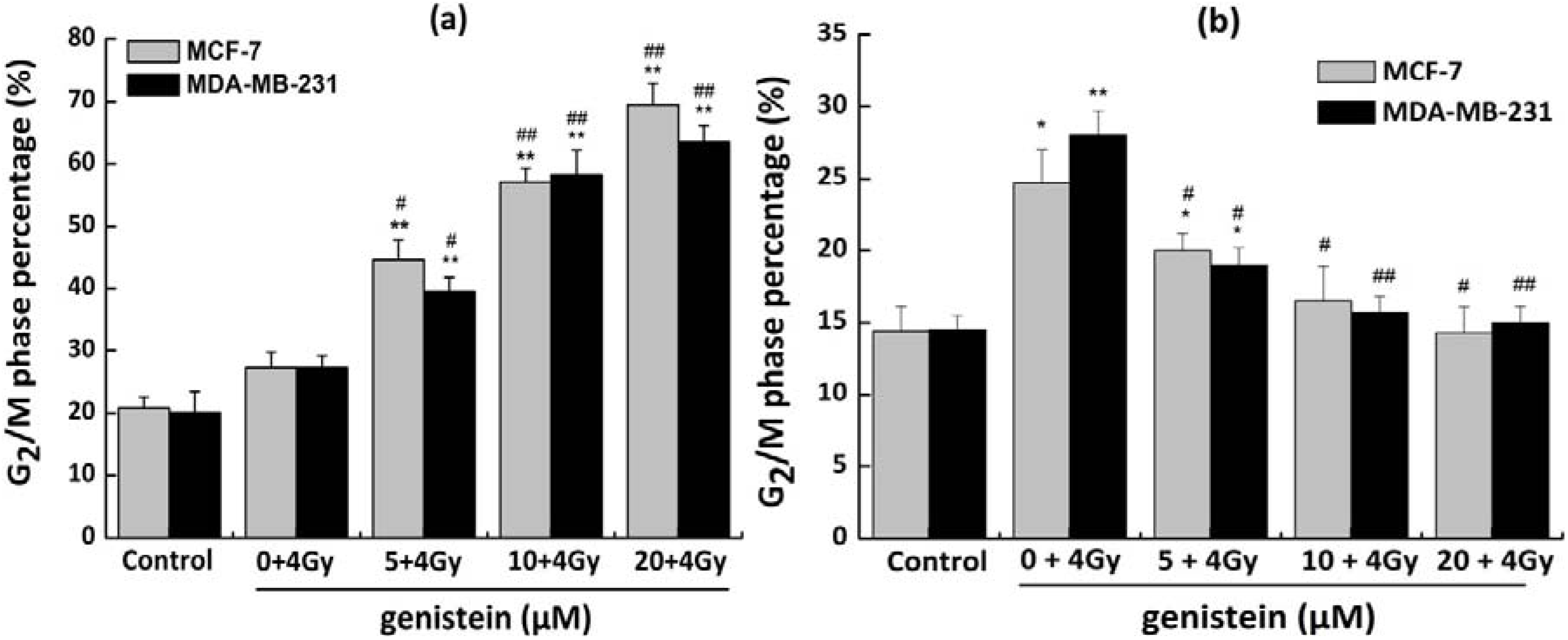
2.4. Genistein Induced G2/M Phase Arrest

2.5. Genistein Pretreatment Followed by Irradiation with X-rays Exacerbated G2/M Phase Arrest
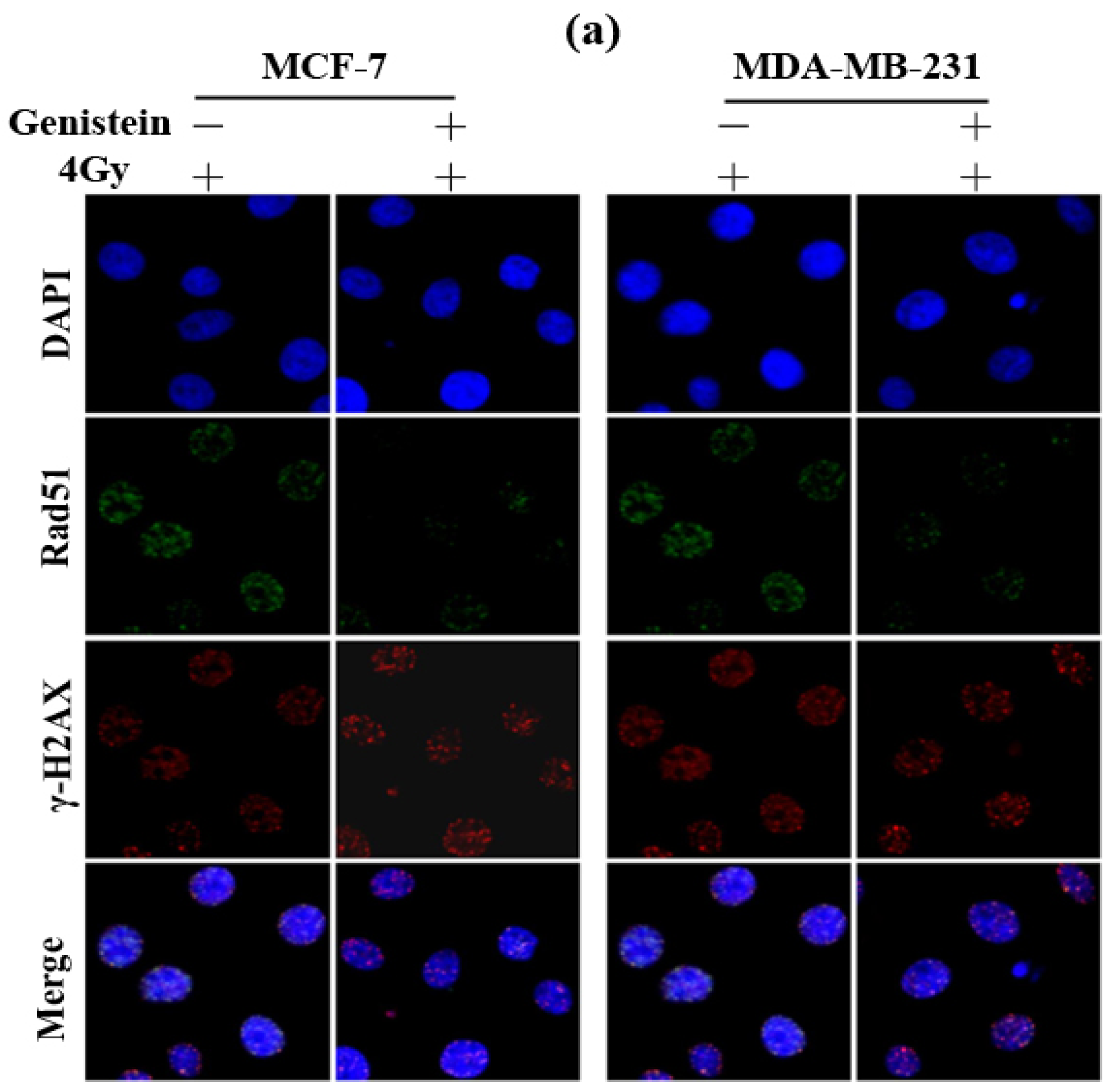
2.6. Genistein Pretreatment Followed by Irradiation with X-rays Inhibited DNA Repair and Increased Cell Apoptosis
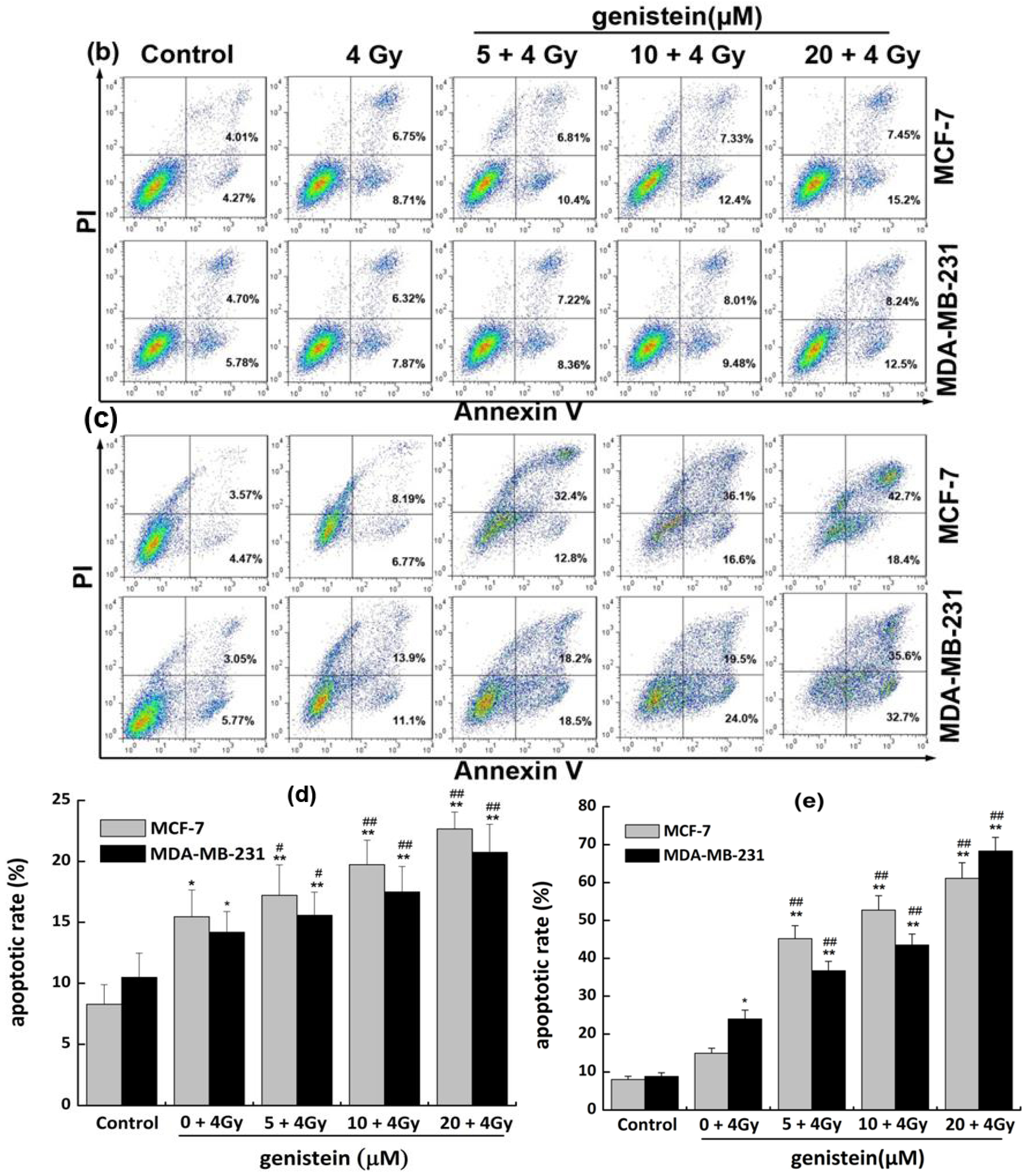
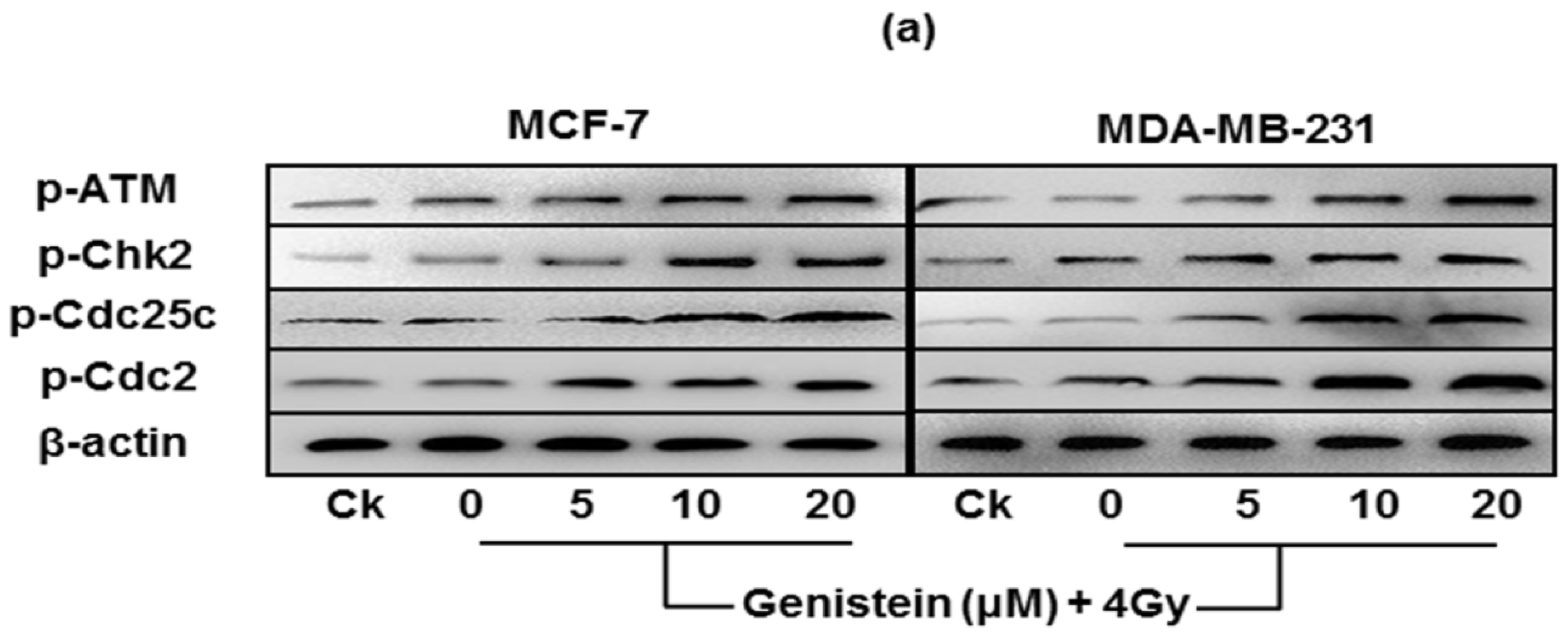
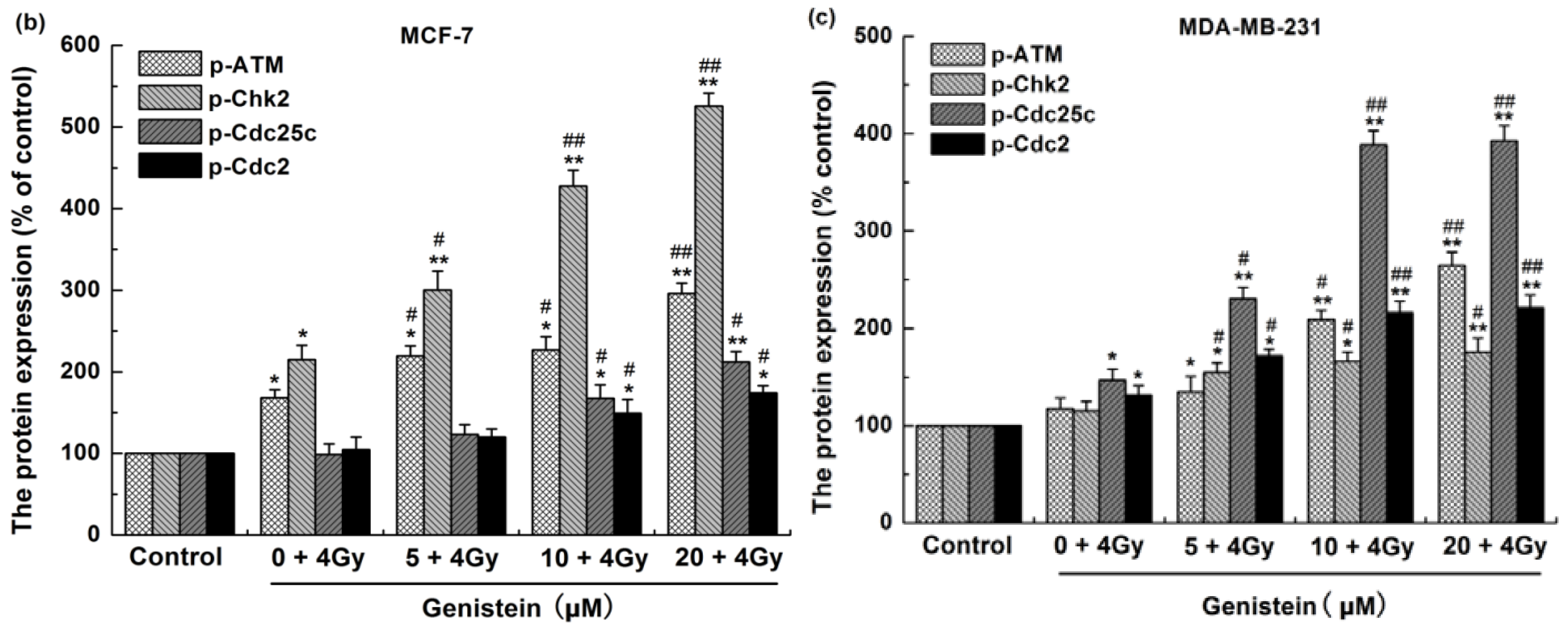
2.7. Genistein Pretreatment Followed by Irradiation with X-rays Activated G2/M Checkpoint Proteins and Affected the Expression of Cell Apoptosis Associated Proteins
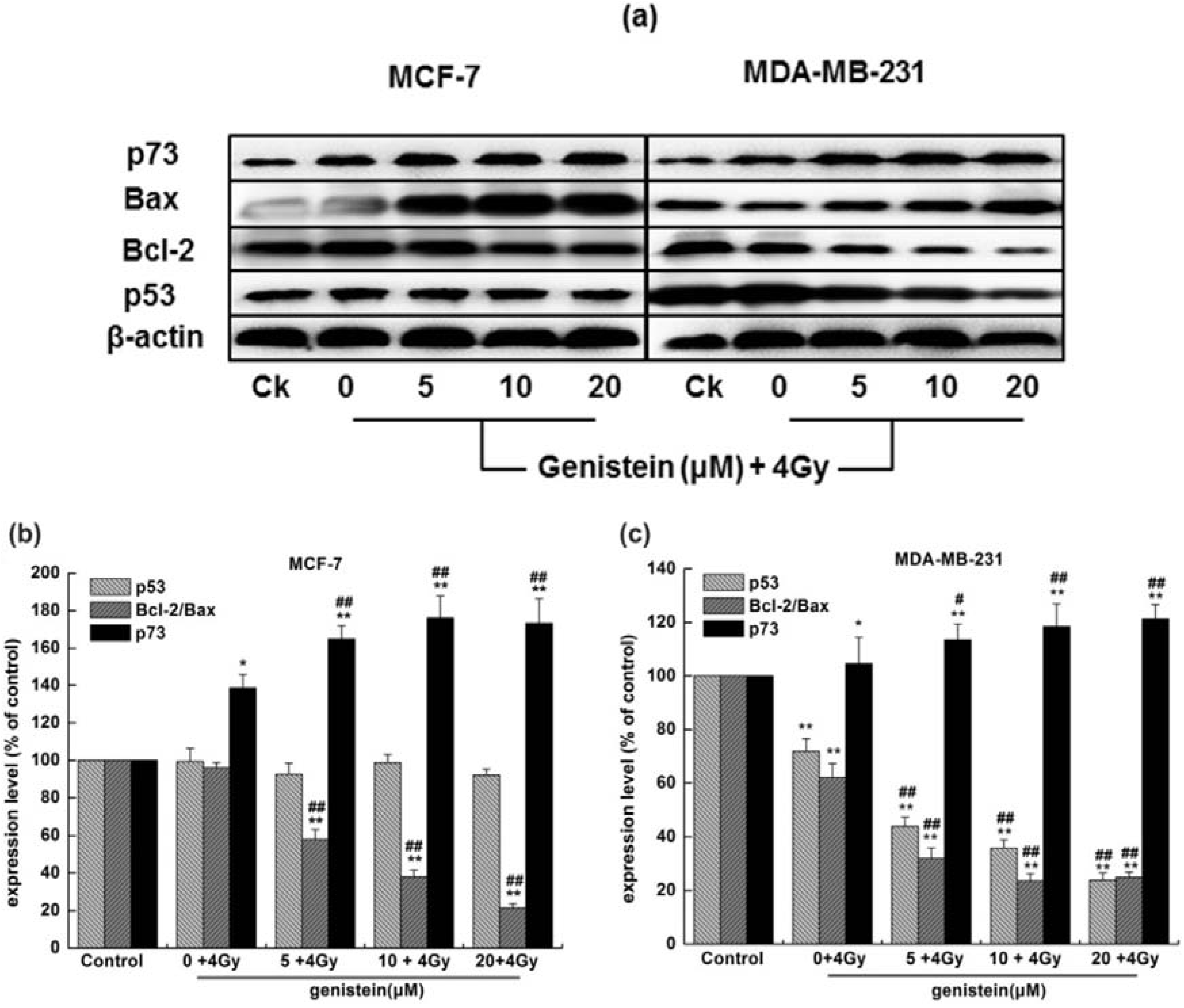
2.8. Discussion
3. Experimental
3.1. Cell Culture and Genistein Treatment
3.2. Irradiation
3.3. MTT Proliferation Assay
3.4. Colony Formation Assay
3.5. γ-H2AX and Rad51 Foci Immunofluorescence
3.6. Cell Cycle Progression Analysis
3.7. Detection of Apoptosis
3.8. Western Blot Analysis
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ER | estrogen receptor |
| ER+ | estrogen receptor positive |
| ER- | estrogen receptor negative |
| Genistein | 5,7,4-trihydroxyisoflavone |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | dimethyl sulfoxide |
| γ-H2AX | phosphorylation of histone H2AX |
| DSB | DNA double-strand breaks |
| PBS | phosphate buffered saline |
| PI | propidium iodide |
| RT | Radiotherapy |
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar]
- Biswas, D.K.; Averboukh, L.; Sheng, S.; Martin, K.; Ewaniuk, D.S.; Jawde, T.F.; Wang, F.; Pardee, A.B. Classification of breast cancer cells on the basis of a functional assay for estrogen receptor. Mol. Med. 1998, 4, 454–467. [Google Scholar]
- Parl, F.F.; Schmidt, B.P.; Dupont, W.D.; Wagner, R.K. Prognostic significance of estrogen receptor status in breast cancer in relation to tumor stage, axillary node metastasis, and histopathologic grading. Cancer 1984, 54, 2237–2242. [Google Scholar] [CrossRef]
- Crowe, J.P., Jr.; Gordon, N.H.; Hubay, C.A.; Shenk, R.R.; Zollinger, R.M.; Brumberg, D.J.; McGuire, W.L.; Shuck, J.M. Estrogen receptor determination and long term survival of patients with carcinoma of the breast. Surg. Gynecol. Obstet. 1991, 173, 273–278. [Google Scholar]
- Anderson, W.F.; Chu, K.C.; Chatterjee, N.; Brawley, O.; Brinton, L.A. Tumor variants by hormone receptor expression in white patients with node-negative breast cancer from the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2001, 19, 18–27. [Google Scholar]
- Zha, L.; Qiao, T.; Yuan, S.; Lei, L. Enhancement of radiosensitivity by CpG-oligodeoxyribonucleotide-7909 in human non-small cell lung cancer A549 cells. Cancer Biother. Radiopharm. 2010, 25, 165–170. [Google Scholar] [CrossRef]
- King, R.W.; Jackson, P.K.; Kirschner, M.W. Mitosis in transition. Cell 1994, 79, 563–571. [Google Scholar] [CrossRef]
- Zhou, B.B.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar]
- Melchionna, R.; Chen, X.B.; Blasina, A.; McGowan, C.H. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat. Cell Biol. 2000, 2, 762–765. [Google Scholar] [CrossRef]
- Chang, C.C.; Hung, C.M.; Yang, Y.R.; Lee, M.J.; Hsu, Y.C. Sulforaphane induced cell cycle arrest in the G2/M phase via the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J. Ovarian Res. 2013, 6, 41. [Google Scholar] [CrossRef]
- Lindqvist, A.; Rodríguez-Bravo, V.; Medema, R.H. The decision to enter mitosis: Feedback and redundancy in the mitotic entry network. J. Cell Biol. 2009, 185, 193–202. [Google Scholar]
- Ando, T.; Kawabe, T.; Ohara, H.; Ducommun, B.; Itoh, M.; Okamoto, T. Involvement of the interaction between p21 and proliferating cell nuclear antigen for the maintenance of G2/M arrest after DNA damage. J. Biol. Chem. 2001, 276, 42971–42977. [Google Scholar]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar]
- Alberts, D.S.; Garcia, D.J. An overview of clinical cancer chemoprevention studies with emphasis on positive phase III studies. J. Nutr. 1995, 125, S692–S697. [Google Scholar]
- Tanos, V.; Brzezinski, A.; Drize, O.; Strauss, N.; Peretz, T. Synergistic inhibitory effects of genistein and tamoxifen on human dysplastic and malignant epithelial breast cells in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 102, 188–194. [Google Scholar] [CrossRef]
- van Duursen, M.B.; Smeets, E.E.; Rijk, J.C.; Nijmeijer, S.M.; van den Berg, M. Phytoestrogens in menopausal supplements induce ER-dependent cell proliferation and overcome breast cancer treatment in an in vitro breast cancer model. Toxicol. Appl. Pharmacol. 2013, 269, 132–140. [Google Scholar] [CrossRef]
- Vandersickel, V.; Depuydt, J.; Van Bockstael, B.; Perletti, G.; Philippe, J.; Thierens, H.; Vral, A. Early increase of radiation-induced γH2AX foci in a human Ku70/80 knockdown cell line characterized by an enhanced radiosensitivity. J. Radiat. Res. 2010, 51, 633–641. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, H.; Dong, G.; Cai, L.; Bai, Y. Chamaejasmine arrests cell cycle, induces apoptosis and inhibits nuclear NF-κB translocation in the human breast cancer cell line MDA-MB-231. Molecules 2013, 18, 845–858. [Google Scholar] [CrossRef]
- Bergamaschi, D.; Samuels, Y.; Jin, B.; Duraisingham, S.; Crook, T.; Lu, X. ASPP1 and ASPP2: Common activators of p53 family members. Mol. Cell. Biol. 2004, 24, 1341–1350. [Google Scholar]
- Sun, Y.; St Clair, D.K.; Xu, Y.A. NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010, 70, 2880–2890. [Google Scholar] [CrossRef]
- Hermann, R.M.; Fest, J.; Christiansen, H.; Hille, A.; Rave-Fränk, M.; Nitsche, M.; Gründker, C.; Viereck, V.; Jarry, H.; Schmidberger, H. Radiosensitization dependent on p53 function in bronchial carcinoma cells by the isoflavone genistein and estradiol in vitro. Strahlenther. Onkol. 2007, 183, 195–202. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Ma, X.F.; Zhou, X.; Gan, L.; Liu, Y.Y.; Wang, Z.H. Isoliquiritigenin enhances radiosensitivity of HepG2 cells via disturbance of redox status. Cell Biochem. Biophys. 2013, 65, 433–444. [Google Scholar] [CrossRef]
- Stoll, B.A. Eating to beat breast cancer: potential role for soy supplements. Ann. Oncol. 1997, 8, 223–225. [Google Scholar] [CrossRef]
- Li, Y.; Upadhyay, S.; Bhuiyan, M.; Sarkar, F.H. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999, 18, 3166–3172. [Google Scholar] [CrossRef]
- Wang, T.T.; Sathyamoorthy, N.; Phang, J.M. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 1996, 17, 271–275. [Google Scholar] [CrossRef]
- Zava, D.T.; Duwe, G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr. Cancer 1997, 27, 31–40. [Google Scholar] [CrossRef]
- Ferguson, D.O.; Sekiguchi, J.M.; Frank, K.M.; Gao, Y.; Sharpless, N.E.; Gu, Y.; Manis, J.; DePinho, R.A.; Alt, F.W. The interplay between nonhomologous end-joining and cell cycle checkpoint factors in development, genomic stability, and tumorigenesis. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 395–403. [Google Scholar] [CrossRef]
- Stucki, M.; Stagljar, I.; Jónsson, Z.O.; Hübscher, U. A coordinated interplay: proteins with multiple functions in DNA replication, DNA repair, cell cycle/checkpoint control, and transcription. Prog. Nucl. Acid Res. Mol. Biol. 2001, 65, 261–298. [Google Scholar]
- Antonsson, B.; Martinou, J.C. The Bcl-2 protein family. Exp. Cell. Res. 2000, 256, 50–57. [Google Scholar] [CrossRef]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef]
- Mori, E.; Takahashi, A.; Yamakawa, N.; Kirita, T.; Ohnishi, T. High LET heavy ion radiation induces p53-independent apoptosis. J. Radiat. Res. 2009, 50, 37–42. [Google Scholar] [CrossRef]
- Stiewe, T. The p53 family in differentiation and tumorigenesis. Nat. Rev. Cancer 2007, 7, 165–168. [Google Scholar] [CrossRef]
- Moll, U.M.; Slade, N. p63 and p73: Roles in development and tumor formation. Mol. Cancer Res. 2004, 2, 371–386. [Google Scholar]
- Nguyen, D.T.; Hernandez-Montes, E.; Vauzour, D.; Schönthal, A.H.; Rice-Evans, C.; Cadenas, E.; Spencer, J.P. The intracellular genistein metabolite 5,7,3',4'-tetrahydroxyisoflavone mediates G2-M cell cycle arrest in cancer cells via modulation of the p38 signaling pathway. Free Radic. Biol. Med. 2006, 41, 1225–1239. [Google Scholar] [CrossRef]
- Leung, H.Y.; Yung, L.H.; Poon, C.H.; Shi, G.; Lu, A.L.; Leung, L.K. Genistein protects against polycyclic aromatic hydrocarbon-induced oxidative DNA damage in non-cancerous breast cells MCF-10A. Brit. J. Nutr. 2009, 101, 257–262. [Google Scholar] [CrossRef]
- Sobottka, S.B.; Berger, M.R. Assessment of antineoplastic agents by MTT assay: Partial underestimation of antiproliferative properties. Cancer Chemother. Pharmacol. 1992, 30, 385–393. [Google Scholar] [CrossRef]
- Vindelov, L.L.; Christensen, I.J.; Nissen, N.I. Standardization of high-resolution flow cytometric DNA analysis by the simultaneous use of chicken and trout red blood cells as internal reference standards. Cytometry 1983, 3, 328–331. [Google Scholar] [CrossRef]
- Severson, R.K.; Nomura, A.M.; Grove, J.S.; Stemmermann, G.N. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989, 49, 1857–1860. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein Enhances the Radiosensitivity of Breast Cancer Cells via G2/M Cell Cycle Arrest and Apoptosis. Molecules 2013, 18, 13200-13217. https://doi.org/10.3390/molecules181113200
Liu X, Sun C, Jin X, Li P, Ye F, Zhao T, Gong L, Li Q. Genistein Enhances the Radiosensitivity of Breast Cancer Cells via G2/M Cell Cycle Arrest and Apoptosis. Molecules. 2013; 18(11):13200-13217. https://doi.org/10.3390/molecules181113200
Chicago/Turabian StyleLiu, Xiongxiong, Chao Sun, Xiaodong Jin, Ping Li, Fei Ye, Ting Zhao, Li Gong, and Qiang Li. 2013. "Genistein Enhances the Radiosensitivity of Breast Cancer Cells via G2/M Cell Cycle Arrest and Apoptosis" Molecules 18, no. 11: 13200-13217. https://doi.org/10.3390/molecules181113200