Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii
Abstract
:1. Introduction
2. Results and Discussion

2.1. Antimicrobial Bioassay
| Sample | Antimicrobial MIC (mg/mL) | ||||
|---|---|---|---|---|---|
| B.s. | E.c. | K.p. | S.a. | C.a. | |
| Myricetrin-3-O-rhamnoside (1) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Quercetin-3-O-rhamnoside (2) | 0.13 | >0.25 | >0.25 | >0.25 | 0.25 |
| Quercetin (3) | 0.03 | >0.25 | 0.25 | >0.25 | 0.02 |
| Trans-N-(p-coumaroyl) serotonin (4) | 0.25 | 0.25 | 0.25 | 0.25 | 0.13 |
| Croton menyharthii crude | 3.13 | 1.56 | 3.13 | 1.56 | 6.25 |
| Croton menyharthii hexane | 0.78 | 0.78 | 1.56 | 0.78 | 0.78 |
| Croton menyharthii DCM | 0.39 | 0.78 | 1.56 | 0.78 | 0.78 |
| Croton menyharthii ethyl acetate | 0.39 | 0.39 | 0.39 | 0.78 | 0.39 |
| Croton menyharthii butanol | 1.56 | 3.13 | 1.56 | 3.13 | 3.13 |
| Neomycin | 1.6 × 10−3 | 0.8 × 10−3 | 0.8 × 10−3 | 1.6 × 10−3 | |
| Amphotericin B | 9.77 × 10−3 | ||||
2.2. Enzyme Inhibition Bioassay Results
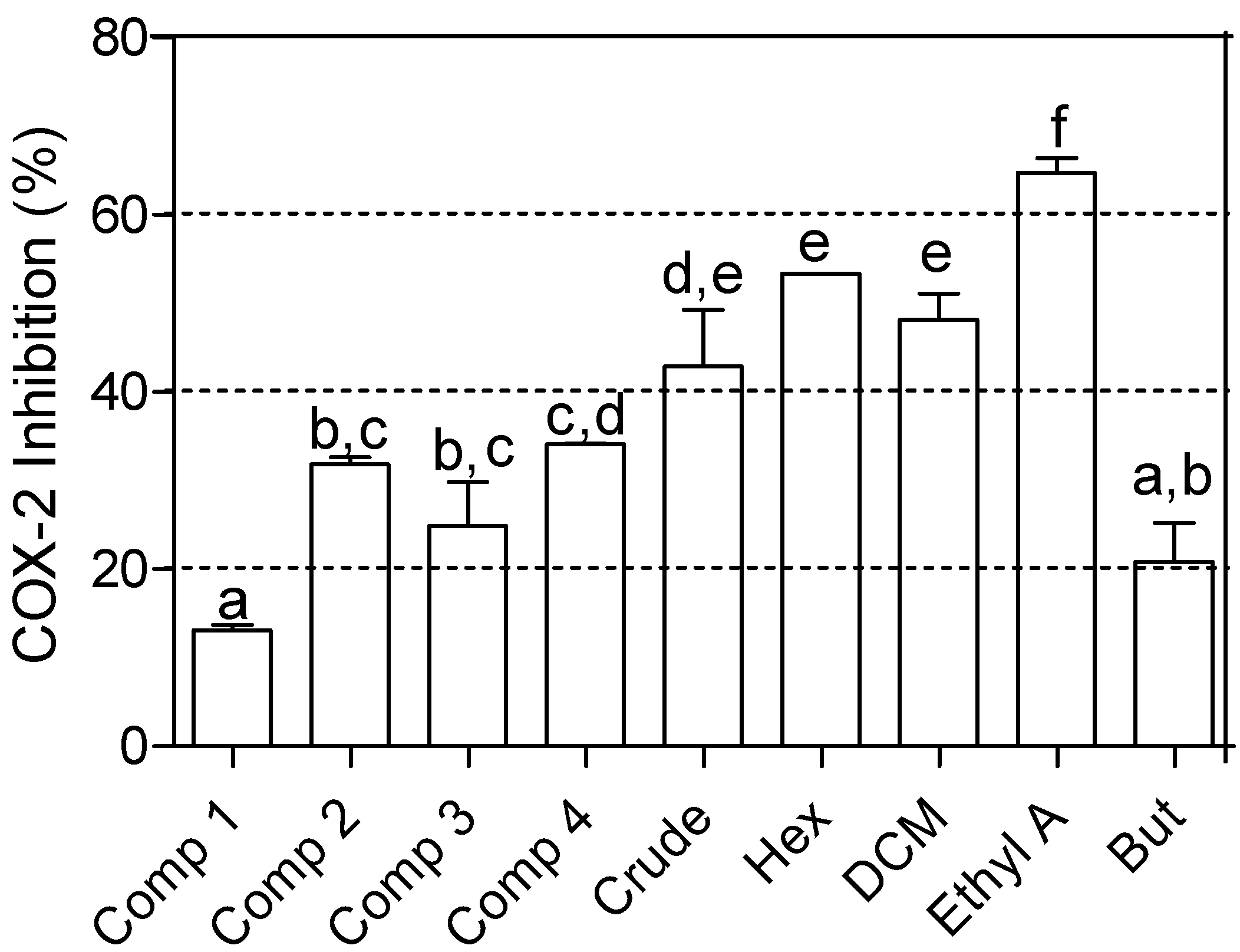
| Sample name | AChE inhibitory activity | α-Glucosidase inhibitory activity | ||
|---|---|---|---|---|
| Percentage inhibition * | IC50 (µg/mL) | Percentage inhibition * | IC50 (µg/mL) | |
| Myricetrin-3-O-rhamnoside (myricetrin) (1) | 54.3 ± 1.1 b | 65.0 ± 7.7 d | 89.1 ± 5.3 e | 79.0 ± 4.3 a,b |
| Quercetin-3-O-rhamnoside (2) | 53.3 ± 1.0 b,c | 60.7 ±7.9 d | 47.0 ± 1.7 a | 122.7 ± 1.6 a,b |
| Quercetin (3) | 56.6 ± 0.3 c | 41.6 ± 6.0 c | 67.1 ± 4.2 b | 30.9 ± 8.4 a,b |
| Trans-N-(p-coumaroyl) serotonin (4) | 72.6 ± 4.9 f | 15.0 ± 0.8 b | 97.3 ± 3.2 f | 5.3 ± 0.3 a |
| Croton menyharthii crude | 48.2 ± 1.8 a | 988.4 ± 12.6 i | 98.3 ± 1.4 f | 43.7 ± 2.2 a,b |
| Croton menyharthii hexane | 63.4 ± 3.4 e | 658.3 ± 8.2 g | 95.1 ± 1.5 f | 55.5 ± 10.5 a,b |
| Croton menyharthii DCM | 60.5 ± 2.2 d | 257.5 ± 9.1 f | 95.6 ± 4.7 f | 47.5 ± 0.4 b,c |
| Croton menyharthii ethyl acetate | 81.4 ± 1.6 g | 105.0 ± 2.0 e | 91.1 ± 2.2 e | 90.3 ± 0.9 a,b |
| Croton menyharthii butanol | 52.7 ± 0.8 b | 768.6 ± 5.6 h | 72.1 ± 3.5 c | 366.3 ± 107.1 c |
| Galanthamine | 88.3 ± 2.0 h | 0.3 ± 0.1 a | ||
| Acarbose | 83.6 ± 2.6 d | 103.3 ± 9.3 a,b | ||
3. Experimental
3.1. General
3.2. Plant Material-Collection and Authentication
3.2.1. Extraction
3.2.2. Solvent Partitioning of the Crude Extracts
3.3. Isolation of Compounds from C. menyharthii EtOAc Extract
3.4. Structure Elucidation of the Compounds
3.5. Antimicrobial Bioassays
3.5.1. Antibacterial Microdilution Bioassay
3.5.2. Antifungal Microdilution Bioassay
3.6. Enzyme Inhibition Bioassays
3.6.1. Cyclooxygenase (COX-2) Inhibitory Bioassay
3.6.2. Acetylcholinesterase (AChE) Inhibitory Bioassay
3.6.3. α-Glucosidase Inhibitory Activity
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Samuelsson, G.; Farah, M.H.; Claeson, P.; Hagos, M.; Thulin, M.; Hedberg, O.; Warfa, A.M.; Hassan, A.O.; Elmi, A.H.; Abdurahman, A.D.; Ehmi, A.S.; Abdi, Y.A.; Alin, M.H. Inventory of plants used in traditional medicine in Somalia. II. Plants of the families Combretaceae to Labiatae. J. Ethnopharmacol. 1982, 37, 47–70. [Google Scholar]
- Elmi, A.S.; Svendsen, A.B.; Scheffer, J.C.; Verpoorte, R. Screening of some Somalian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 1986, 17, 283–288. [Google Scholar] [CrossRef]
- Mavundza, E.J.; Maharaj, R.; Finnie, J.F.; Kabera, G.; Van Staden, J. An ethnobotanical survey of mosquito repellent plants in uMkhanyakude district, KwaZulu-Natal province, South Africa. J. Ethnopharmacol. 2011, 137, 1516–1520. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpotzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 40, 4168–4170. [Google Scholar]
- Wang, Y.; Liu, J.; Chen, F.; Zhao, G. Effects of molecular structure of polyphenols on their non-covalent interactions with oat β-glucan. J. Agric. Food Chem. 2013, 61, 4533–4538. [Google Scholar]
- Taylor, J.L.S.; Van Staden, J. COX-1 inhibitory activity in extracts from Eucomis L’Herit. species. J. Ethnopharmacol. 2001, 75, 257–265. [Google Scholar] [CrossRef]
- Gbolade, A.A. Inventory of antidiabetic plants in selected districts of Lagos State, Nigeria. J. Ethnopharmacol. 2009, 121, 135–139. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; di Naso, F.C.; Porawski, M.; Marcolin, E.; Kretzmann, N.A.; Falcão Ferraz, A-B.; Richter, M.F.; Marroni, C.A.; Marroni, N.P. Treatment with aqueous extract from Croton cajucara benth reduces hepatic oxidative stress in streptozotocin-diabetic rats. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Okokon, J.E.; Nwafor, P.A.; Umoh, E.E.; Okokon, P.J.; Udobang, J.A. Antidiabetic and hypolipidemic of ethanolic root extract of Croton zambesicus on alloxan induced diabetic rats. Asian J. Phar. Biol. Res. 2011, 1, 493–499. [Google Scholar]
- Torrico, F.; Cepeda, M.; GuerreroI, G.; Melendez, F.; BlancoI, Z.; Canelón, D.J.; Diaz, B.; Compagnone, R.S.; Suárez, A.I. Hypoglycaemic effect of Croton cuneatus in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2007, 17, 166–169. [Google Scholar] [CrossRef]
- Shibano, M.; Tsukamoto, D.; Masuda, A.; Tanaka, Y.; Kusano, G. Two new pyrroline alkaloids, radicamines A and B, as inhibitors of α-glucosidase from Lobelia chinensis Lour. Chem. Pharm. Bull. 2001, 49, 1362–1365. [Google Scholar] [CrossRef]
- Choudharya, M.I.; Rasheeda, S.A.A.; Marasinia, B.P.; Hussaina, N.; Kaleemc, A.W.; Rahmana, A. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw as novel inhibitors of α-glucosidase enzyme and protein glycation. Phytochemistry 2011, 4, 404–406. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.-J.; Song, H.-C. Alpha-glucosidase and alpha-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Pereira, D.F.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.R.B.; Guedes, A.; Pizzolatti, M.G.; Barreto Silva, F.R.M. Effects of flavonoids on α-glucosidase activity: Potential targets for glucose homeostasis. Nutrition 2011, 27, 1161–1167. [Google Scholar] [CrossRef]
- Fossen, T.; Larsen, Å.; Kiremire, B.T.; Andersen, Ø.M. Flavonoids from blue flowers of Nymphaea caerulèa. Phytochemistry 1999, 51, 1133–1137. [Google Scholar] [CrossRef]
- Wawer, I.; Zielinska, A. 13C CP/MAS NMR studies of flavonoids. Magn. Reson. Chem. 2001, 39, 374–380. [Google Scholar] [CrossRef]
- Watanabe, M. Antioxidative phenolic compounds from Japanese Barnyard Millet (Echinochloautilis) grains. J. Agric. Food Chem. 1999, 47, 4500–4505. [Google Scholar]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Masoko, P.; Picard, J.; Eloff, J.N. The antifungal activity of twenty-four southern Africa Combretum species (Combretaceae). S. Afri. J. Bot. 2007, 73, 173–183. [Google Scholar] [CrossRef]
- Zschocke, S.; Van Staden, J. Cryptocarya species-substitute plants for Ocotea bullata? A pharmacological investigation in terms of cyclooxygenase-1 and -2 inhibitions. J. Ethnopharmacol. 2000, 71, 473–478. [Google Scholar] [CrossRef]
- Ellman, G.L.; Coutney, D.; Andies, V.; Featherstone, R.M. A new and rapid colourimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Tao, T.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatog. 2013, 27, 148–155. [Google Scholar] [CrossRef]
- Rengasamy, R.R.K.; Aderogba, M.; Amoo, S.O.; Stirk, W.A.; Van Staden, J. Potential antiradical and alpha glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chem. 2013, 141, 1412–1415. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the crude extracts, solvent fractions and isolated compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aderogba, M.A.; Ndhlala, A.R.; Rengasamy, K.R.R.; Van Staden, J. Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii. Molecules 2013, 18, 12633-12644. https://doi.org/10.3390/molecules181012633
Aderogba MA, Ndhlala AR, Rengasamy KRR, Van Staden J. Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii. Molecules. 2013; 18(10):12633-12644. https://doi.org/10.3390/molecules181012633
Chicago/Turabian StyleAderogba, Mutalib A., Ashwell R. Ndhlala, Kannan R. R. Rengasamy, and Johannes Van Staden. 2013. "Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii" Molecules 18, no. 10: 12633-12644. https://doi.org/10.3390/molecules181012633
APA StyleAderogba, M. A., Ndhlala, A. R., Rengasamy, K. R. R., & Van Staden, J. (2013). Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii. Molecules, 18(10), 12633-12644. https://doi.org/10.3390/molecules181012633




