Qualitative and Quantitative Analysis of Andrographis paniculata by Rapid Resolution Liquid Chromatography/Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Sample Preparation and Chromatographic Conditions
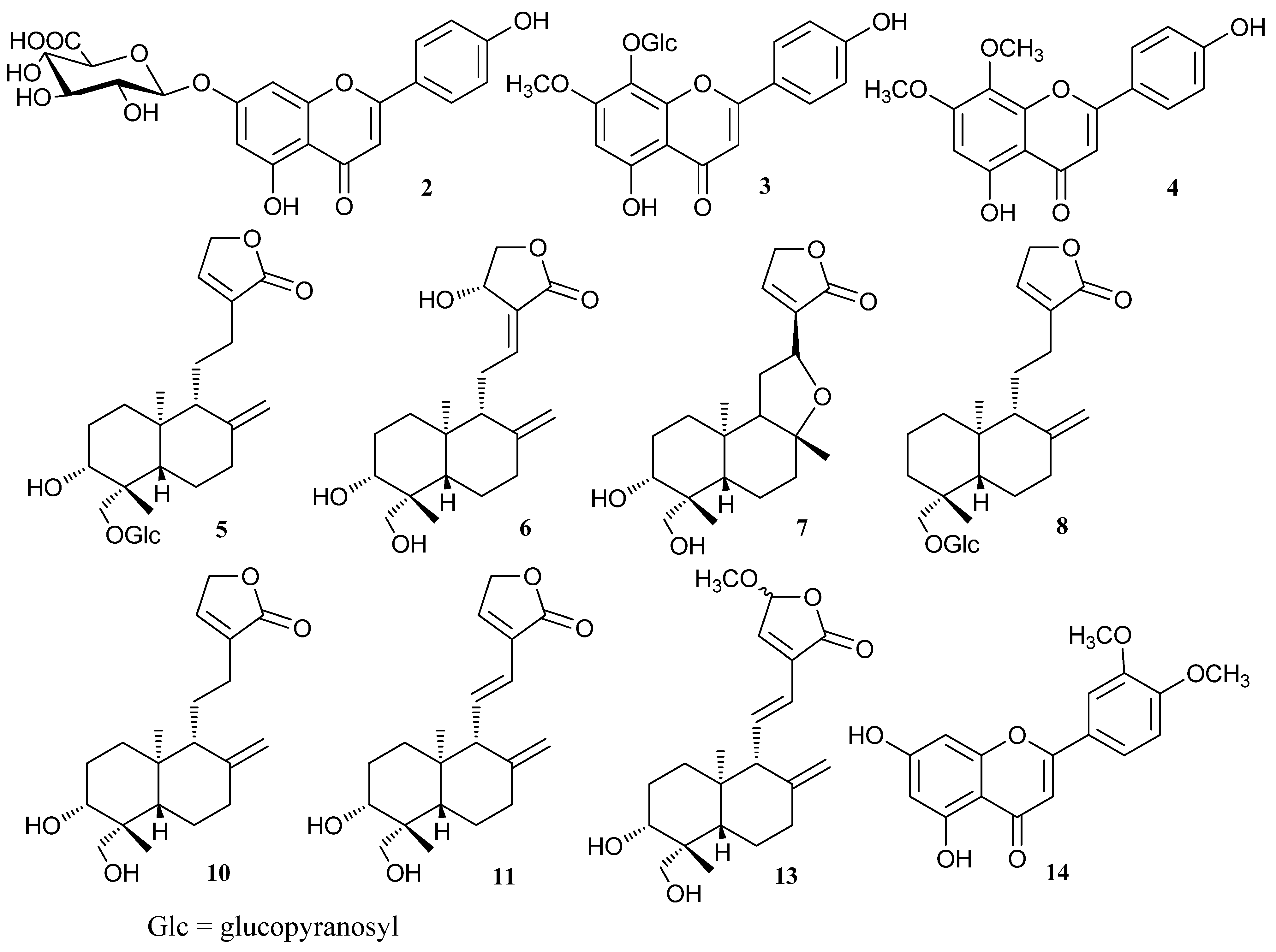

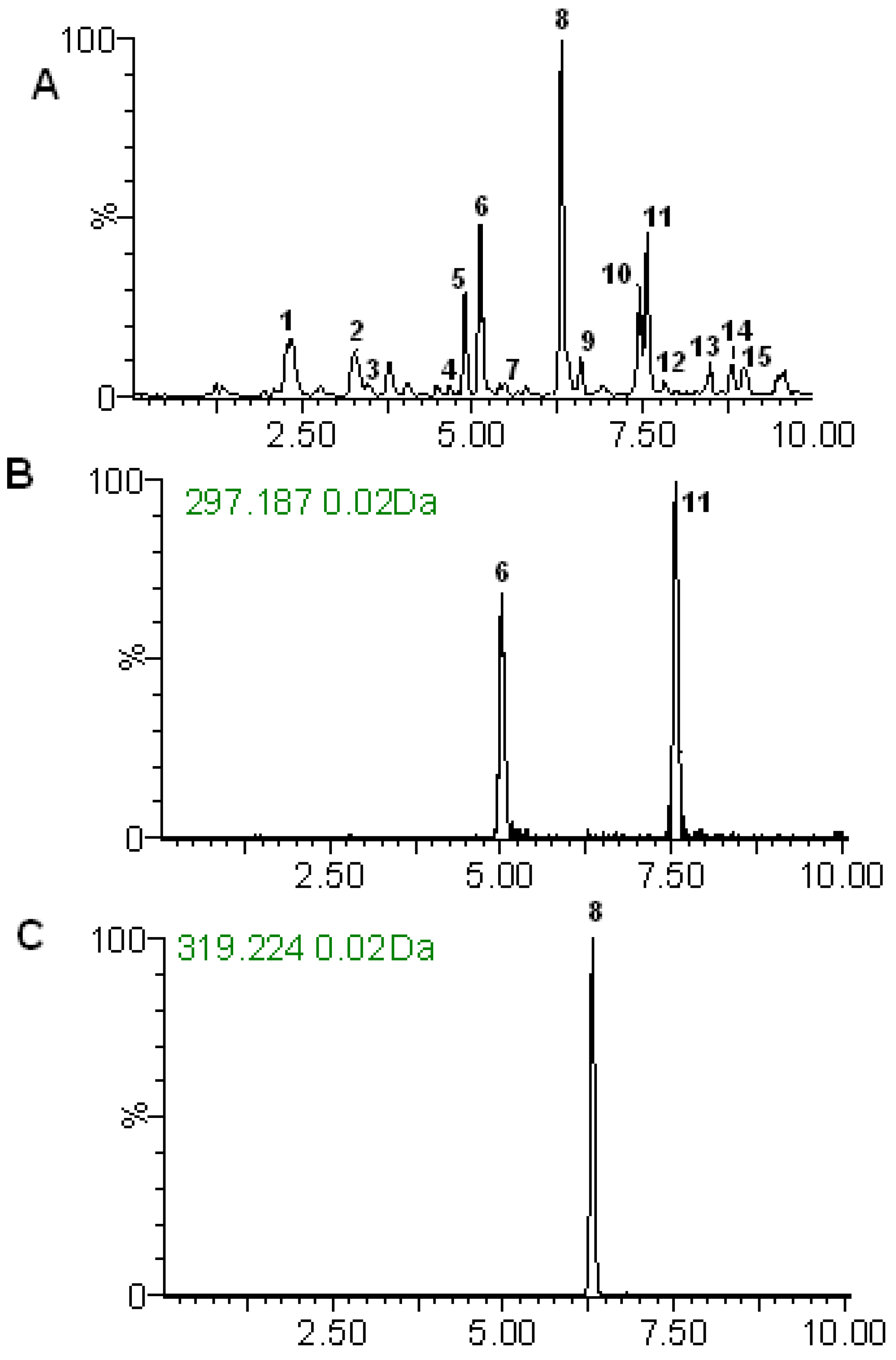
2.2. RRLC-TOF Analysis
2.2.1. Identification of Diterpene Lactones
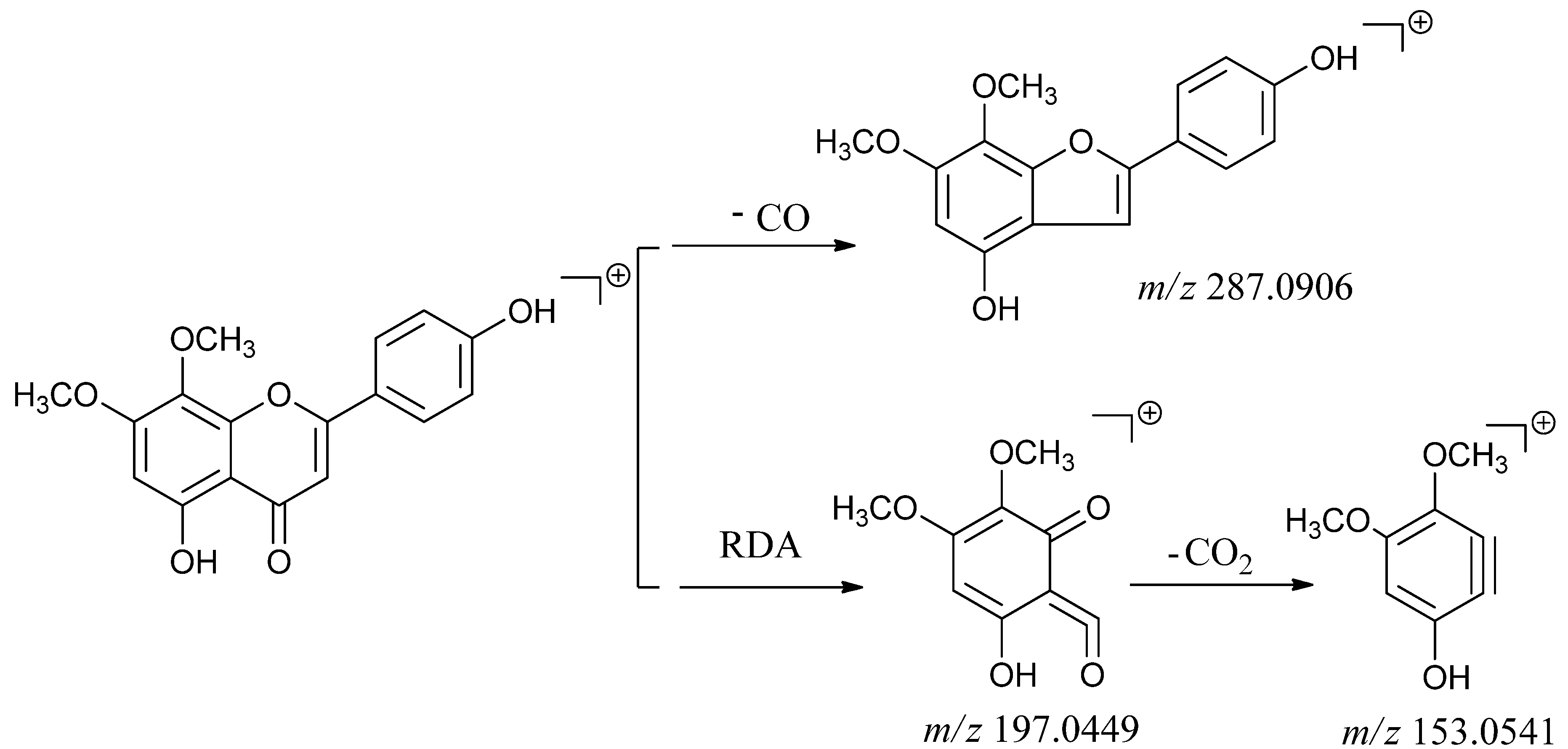
2.2.2. Identification of Flavone Compounds
| No. | tR (min) | MS and MS/MS (m/z) | Type | Identification |
|---|---|---|---|---|
| 1 | 2.33 | [M+H]+ 463.3319 | Flavone | Unknown |
| [M+H-H2O]+ 445.3206 | ||||
| [Aglycone+H]+ 287.0592 | ||||
| 2 | 3.28 | [M+H]+ 447.0887 | Flavone | Apigenin-7-O-β-D-glucuronide |
| Calculated [M+H]+ 447.0927 | ||||
| [2M+H]+893.1624 | ||||
| [Aglycone+H]+ 271.0568 | ||||
| 3 | 3.49 | [M+H]+463.1211 | Flavone | (5,4'-dihydroxy-7-methoxy-8-O-β-d-glucopyrarosyl-flavone |
| Calculated [M+H]+ 463.1240 | ||||
| [M+H-H2O]+445.3185 | ||||
| [Aglycone+H]+ 301.0673 | ||||
| 4 | 4.67 | [M+H]+ 315.0859 | Flavone | 5,4'-dihydroxy-7,8-dimethoxy-flavone |
| Calculated [M+H]+ 315.0869 | ||||
| [M+H-CO]+ 287.1990 | ||||
| [M+H-2CH3]+285.1879 | ||||
| [M+H-CH3]+ 300.0630 | ||||
| Typical fragment 287.2906 | ||||
| Typical fragment 197.0449 | ||||
| Typical fragment 153.0941 | ||||
| 5 | 4.89 | [M+H]+ 497.2740 | Diterpene | 14-deoxyandrographiside |
| Calculated [M+H]+ 497.2751 | ||||
| [M+H-Glc]+ 335.2223 | ||||
| [M+H-Glc-H2O]+ 317.2087 | ||||
| [M+H-Glc-2H2O]+299.1959 | ||||
| Typical fragment 287.1954 | ||||
| Typical fragment 259.1660 | ||||
| [M+Na]+ 519.2543 | ||||
| [2M+H]+ 993.5358 | ||||
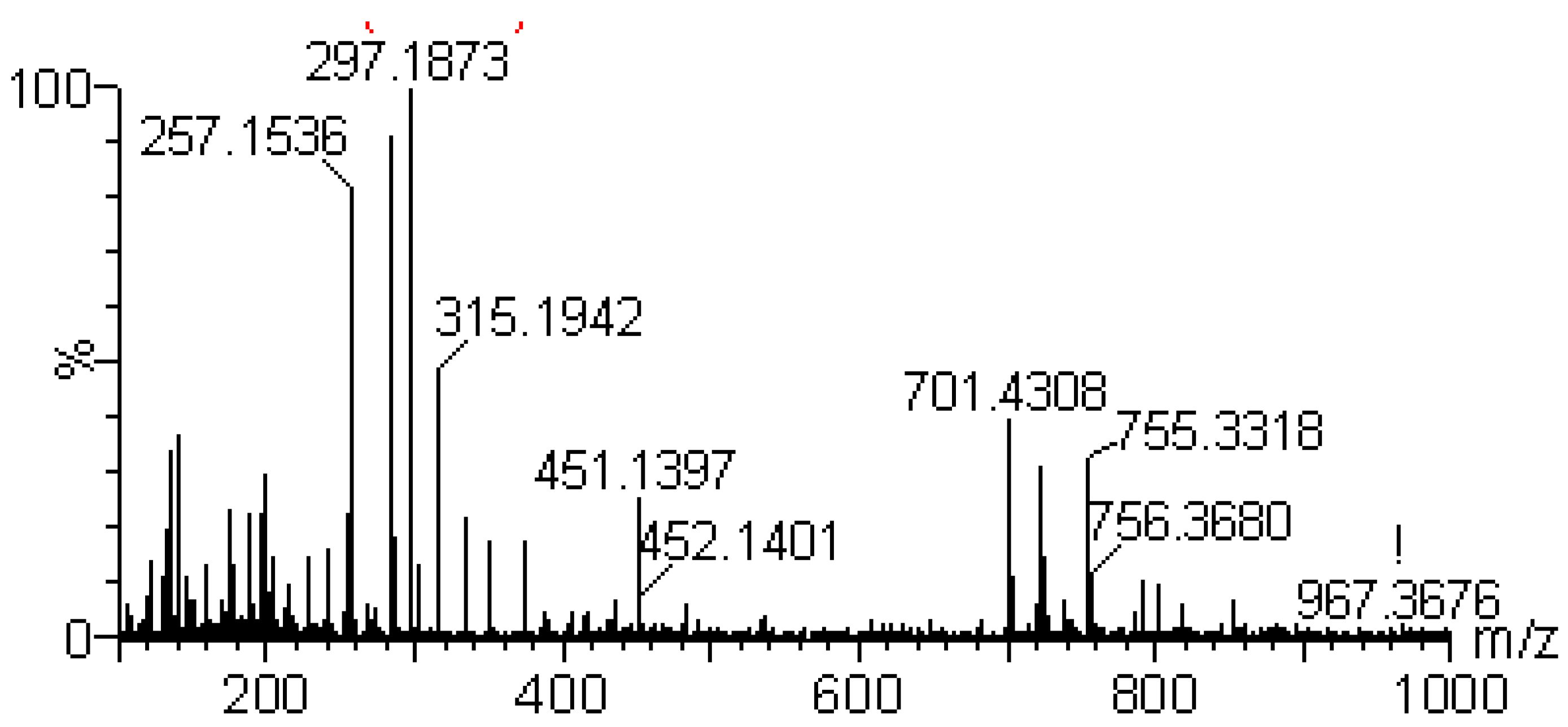
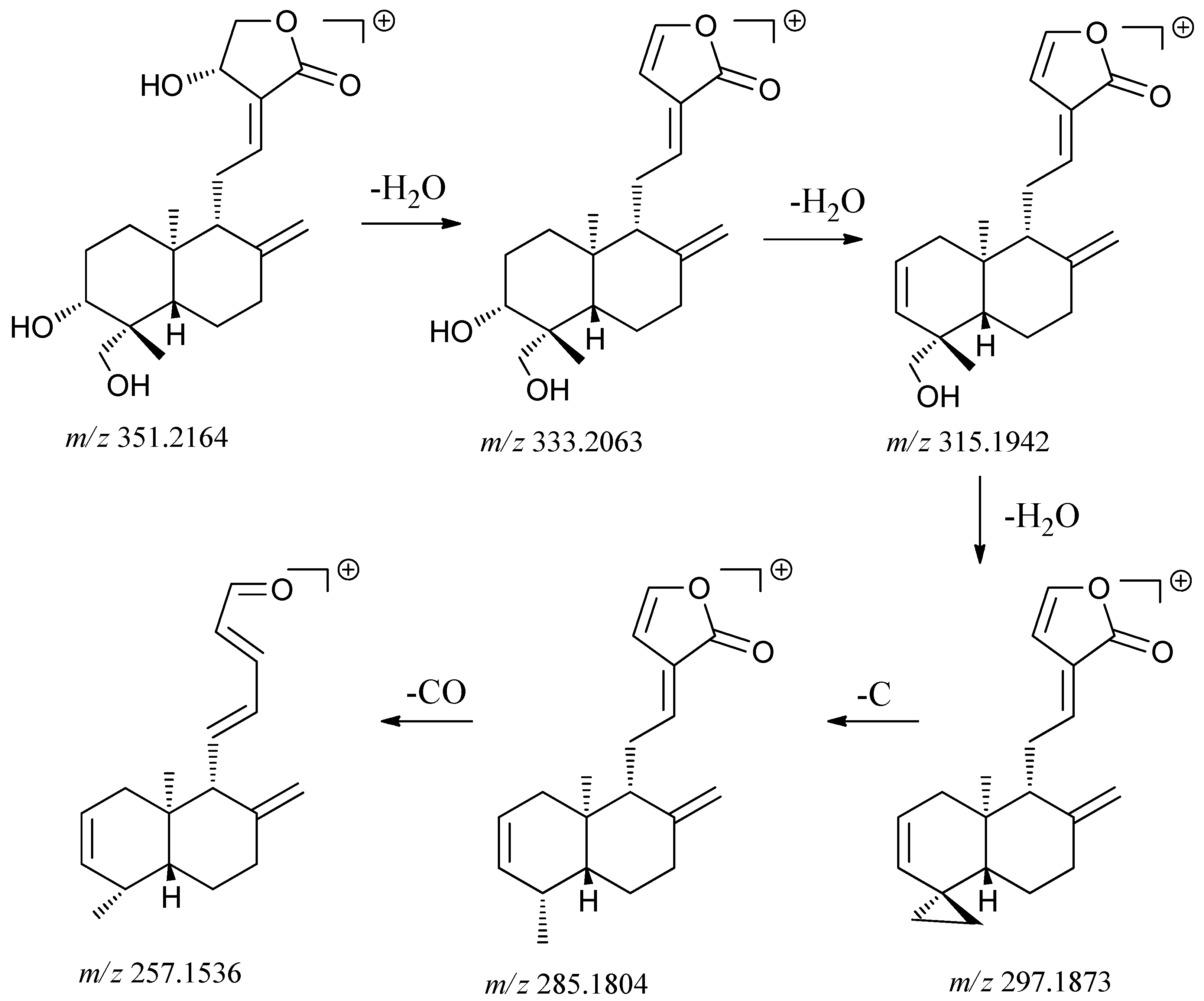
| 6 | 5.11 | [M+H]+351.2164 | Diterpene | Andrographolide |
| Calculated [M+H]+ 351.2171 | ||||
| [M+H-H2O]+ 333.2063 | ||||
| [M+H-2H2O]+ 315.1929 | ||||
| [M+H-3H2O]+ 297.1798 | ||||
| Typical fragment 285.1804 | ||||
| Typical fragment 257.1501 | ||||
| [2M+H]+ 701.4224 | ||||
| 7 | 5.40 | [M+H]+351.2173 | Diterpene | Isoandrographolide |
| Calculated [M+H]+ 351.2171 | ||||
| [M+H-H2O]+ 333.2065 | ||||
| [M+H-2H2O]+ 315.1954 | ||||
| [M+H-3H2O]+ 297.1828 | ||||
| Typical fragment 285.1847 | ||||
| Typical fragment 257.1558 | ||||
| [2M+H]+ 701.4210 | ||||
| 8 | 6.30 | [M+H]+ 481.2825 | Diterpene | Neoandrographolide |
| Calculated [M+H]+ 481.2801 | ||||
| [M+H-Glc]+ 319.2288 | ||||
| [M+H-Glc-H2O]+ 301.2125 | ||||
| Typical fragment 289.2166 | ||||
| Typical fragment 261.1866 | ||||
| [M+Na]+ 503.2672 | ||||
| [M+NH4]+ 498.3100 | ||||
| [2M+H]+ 961.5472 | ||||
| 9 | 6.60 | [M+H]+ 379.2119 | Diterpene | Unknown |
| [M+Na]+ 401.1998 | ||||
| [M+K]+ 417.1768 | ||||
| [M+H-H2O]+ 361.2029 | ||||
| [M+H-2H2O]+ 343.1904 | ||||
| [M+H-2H2O-CH2]+ 329.1776 | ||||
| [M+H-3H2O-CH2]+ 311.1636 | ||||
| Typical fragment 283.1701 | ||||
| Typical fragment 257.1414 | ||||
| [2M+H]+ 757.4134 | ||||
| 10 | 7.44 | [M+H]+ 335.2202 | Diterpene | 14-deoxyandrographolide |
| Calculated [M+H]+ 335.2222 | ||||
| [M+Na]+ 357.2104 | ||||
| [M+H-H2O]+ 317.2092 | ||||
| [M+H-2H2O]+ 299.1965 | ||||
| Typical fragment 287.1896 | ||||
| Typical fragment 259.1652 | ||||
| [2M+H]+ 669.4372 | ||||
| 11 | 7.57 | [M+H]+ 333.2075 | Diterpene | Dehydroandrographolide |
| Calculated [M+H]+ 333.2066 | ||||
| [M+H-H2O]+ 315.1942 | ||||
| [M+H-2H2O]+ 297.1787 | ||||
| Typical fragment 285.1805 | ||||
| Typical fragment 257.1506 | ||||
| [2M+H]+ 665.4048 | ||||
| 12 | 7.83 | [M+H]+ 681.3962 | Diterpene | Unknown© |
| [M+Na]+ 703.3758 | ||||
| [M+H- H2O]+ 663.3945 | ||||
| [M+H-2H2O]+ 645.3752 | ||||
| [M+H-3H2O]+ 627.3672 | ||||
| [M+H-4H2O]+ 609.3548 | ||||
| Typical fragment 297.1833 | ||||
| Typical fragment 269.1812 | ||||
| 13 | 8.48 | [M+H]+ 363.2169 | Diterpene | 3,19-Dihydroxy-15-methoxy-ent-labda-8(17),11,13-trien-16,15-olide |
| Calculated [M+H]+ 363.2171 | ||||
| [M+Na]+ 385.1991 | ||||
| [M+H- H2O]+ 345.2104 | ||||
| [M+H-2H2O]+ 327.1988 | ||||
| [M+H-2H2O-CH2]+ 313.1779 | ||||
| [M+H-3H2O-CH2]+ 295.1685 | ||||
| Typical fragment 283.1694 | ||||
| Typical fragment 255.1414 | ||||
| [2M+H]+ 725.4254 | ||||
| 14 | 8.80 | [M+H]+315.0829 | Flavone | Dihydroxydimethoxyflavone |
| Calculated [M+H]+ 315.0869 | ||||
| [M+H-CO]+ 287.1985 | ||||
| [M+H-CH3]+ 300.0635 | ||||
| Typical fragment 271.0628 | ||||
| Typical fragment 197.0524 | ||||
| 15 | 8.98 | [M+H]+ 697.4302 | Diterpene | Unknown |
| [M+Na]+ 719.4150 | ||||
| [M+H-3H2O-CH2]+ 629.3845 | ||||
| [M+H-4H2O-CH2]+ 611.3734 | ||||
| [M+H-4H2O-CH2-C]+ 599.3704 | ||||
| Typical fragment 297.1829 | ||||
| Typical fragment 255.1491 |
2.3. Methodological Validation of the Quantitative Analysis
| No. | Regression equation | Linear range (μg/mL) | R2 | LODs (μg/mL) | LOQs (μg/mL) |
|---|---|---|---|---|---|
| 6 | y = 22.35x − 9.56 | 0.2–100 | 0.9996 | 0.06 | 0.2 |
| 8 | y = 46.00x − 18.88 | 0.2–100 | 0.9995 | 0.02 | 0.06 |
| 11 | y = 13.25x + 5.813 | 1.0–100 | 0.9998 | 0.06 | 0.2 |
| No. | Source | Geographical regions | Comp. 6 a | Comp. 8 b | Comp. 11 c |
|---|---|---|---|---|---|
| 1 | A. paniculata | Unknown | 0.23 | 0.27 | 0.07 |
| 2 | A. paniculata | Guangxi | 0.65 | 0.73 | 0.80 |
| 3 | A. paniculata | Jiangxi | 0.24 | 0.04 | 0.09 |
| 4 | A. paniculata | Unknown | 1.08 | 0.16 | 0.25 |
| 5 | Andrographis Extract | Sichuan | 6.73 | 11.95 | 10.75 |
| 6 | Andrographis Extract | Sichuan | 3.56 | 9.28 | 16.54 |
| 7 | Andrographis Extract | Sichuan | 7.24 | 9.68 | 17.45 |
| 8 | Andrographis Tablet | Guangxi Fanglue | 1.35 | 0.83 | 0.67 |
| 9 | Andrographis Tablet | Heilongjiang Wusulijiang | 0.33 | 0.42 | 0.52 |
| 10 | Andrographis Tablet | Guangdong Boluoxianfeng | 1.61 | 0.63 | 0.69 |
3. Experimental
3.1. Chemicals and Reagents
3.2. Analytical Sample Preparation
3.3. RRLC-TOF Apparatus and Conditions
3.4. Standard Preparation and Calibration Curves
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chao, W.W.; Kuo, Y.H.; Lin, B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by N-F-κB transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef]
- State Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China (2010 version). People’s Medical Publishing House: Beijing, China, 2010. [Google Scholar]
- Melchior, J.; Spasov, A.; Ostrovskij, O.; Bulanov, A.; Wikman, G. Double-blind, placebo-controlled pilot and Phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine 2000, 7, 341–350. [Google Scholar] [CrossRef]
- Gabrielian, E.; Shukarian, A.; Goukasova, G.; Chandanian, G.; Panossian, A.; Wikman, G.; Wagner, H. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 2002, 9, 589–597. [Google Scholar] [CrossRef]
- Wiart, C.; Kumar, K.; Yusof, M.; Hamimah, H.; Fauzi, Z.; Sulaiman, M. Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phytother. Res. 2005, 19, 1069–1070. [Google Scholar]
- Koteswara Rao, Y.; Vimalamma, G.; Venkata Rao, C.; Tzeng, Y.-M. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry 2004, 65, 2317–2321. [Google Scholar] [CrossRef]
- Smith, P.L.; Maloney, K.N.; Pothen, R.G.; Clardy, J.; Clapham, D.E. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J. Biol. Chem. 2006, 281, 29897–29904. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, H.; Wang, R.; Zhou, K.; Jing, Y.; Qiu, F. Ent-labdane diterpenoid lactone stereoisomers from Andrographis paniculata. J. Nat. Prod. 2008, 71, 852–855. [Google Scholar] [CrossRef]
- Chen, L.X.; Qiu, F.; Wei, H.; Qu, G.X.; Yao, X.S. Nine new ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Helv. Chim. Acta 2006, 89, 2654–2664. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lii, C.K.; Hsu, Y.T.; Lu, C.Y.; Liu, K.L.; Li, C.C.; Chen, H.W. Induction of heme oxygenase-1 and inhibition of TPA-induced matrix metalloproteinase-9 expression by andrographolide in MCF-7 human breast cancer cells. Carcinogenesis 2013, 34, 1843–1851. [Google Scholar] [CrossRef]
- Chen, W.; Feng, L.; Nie, H.; Zheng, X. Andrographolide induces autophagic cell death in human liver cancer cells through cyclophilin D-mediated mitochondrial permeability transition pore. Carcinogenesis 2012, 33, 2190–2198. [Google Scholar] [CrossRef]
- Guan, S.P.; Tee, W.; Ng, D.S.; Chan, T.K.; Peh, H.Y.; Ho, W.E.; Cheng, C.; Mak, J.C.; Wong, W.S. Andrographolide protects against cigarette smoke-induced oxidative lung injury via augmentation of Nrf2 activity. Br. J. Pharmacol. 2013, 168, 1707–1718. [Google Scholar] [CrossRef]
- Jayakumar, T.; Hsieh, C.Y.; Lee, J.J.; Sheu, J.R. Experimental and clinical pharmacology of Andrographis paniculata and Its major bioactive phytoconstituent andrographolide. Evid. Based Complement. Alternat. Med. 2013, 2013, 846740. [Google Scholar]
- Shen, T.; Yang, W.S.; Yi, Y.S.; Sung, G.H.; Rhee, M.H.; Poo, H.; Kim, M.Y.; Kim, K.W.; Kim, J.H.; Cho, J.Y. AP-1/IRF-3 targeted anti-Inflammatory activity of andrographolide isolated from Andrographis paniculata. Evid. Based Complement. Alternat. Med. 2013, 2013, 210736. [Google Scholar]
- Uttekar, M.M.; Das, T.; Pawar, R.S.; Bhandari, B.; Menon, V.; Nutan; Gupta, S.K.; Bhat, S.V. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012, 56, 368–374. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, D.X.; Zhang, W.; Liao, X.Q.; Guan, X.; Bo, H.; Sun, J.Y.; Huang, N.W.; He, J.; Zhang, Y.K.; Tong, J.; Li, C.Y. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kappaB. PLoS One 2013, 8, e56407. [Google Scholar]
- Chen, L.; Jin, H.; Ding, L.; Zhang, H.; Wang, X.; Wang, Z.; Li, J.; Qu, C.; Wang, Y.; Zhang, H. On-line coupling of dynamic microwave-assisted extraction with high-performance liquid chromatography for determination of andrographolide and dehydroandrographolide in Andrographis paniculata Nees. J. Chromatogr. A 2007, 1140, 71–77. [Google Scholar]
- Cheung, H.Y.; Cheung, C.S.; Kong, C.K. Determination of bioactive diterpenoids from Andrographis paniculata by micellar electrokinetic chromatography. J. Chromatogr. A 2001, 930, 171–176. [Google Scholar]
- Pholphana, N.; Rangkadilok, N.; Thongnest, S.; Ruchirawat, S.; Ruchirawat, M.; Satayavivad, J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm.f.) Nees. Phytochem. Anal. 2004, 15, 365–371. [Google Scholar] [CrossRef]
- Qizhen, D.; Jerz, G.; Winterhalter, P. Separation of andrographolide and neoandrographolide from the leaves of Andrographis paniculata using high-speed counter-current chromatography. J. Chromatogr. A 2003, 984, 147–151. [Google Scholar]
- Srivastava, A.; Misra, H.; Verma, R.K.; Gupta, M.M. Chemical fingerprinting of Andrographis paniculata using HPLC, HPTLC and densitometry. Phytochem. Anal. 2004, 15, 280–285. [Google Scholar] [CrossRef]
- Xu, T.; Pan, J.; Zhao, L. Simultaneous determination of four andrographolides in Andrographis paniculata Nees by silver ion reversed-phase high-performance liquid chromatography. J. Chromatogr. Sci. 2008, 46, 747–750. [Google Scholar] [CrossRef]
- Xu, X.Q.; Hu, G.L.; Shen, J.C.; Li, Q.; Wang, X.R. Determination of andrographolide and dehydroandrographolide in Andrographis paniculata nees materials and related patent medicines by reversed-phase high performance liquid chromatography. Se Pu 2002, 20, 446–448. [Google Scholar]
- Zhao, J.; Yang, G.; Liu, H.; Wang, D.; Song, X.; Chen, Y. Determination of andrographolide, deoxyandrographolide and neoandrographolide in the Chinese herb Andrographis paniculata by micellar electrokinetic capillary chromatography. Phytochem. Anal. 2002, 13, 222–227. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.; Kong, L. Quantitative analysis of four major diterpenoids in Andrographis paniculata by 1H NMR and its application for quality control of commercial preparations. J. Pharm. Biomed. Anal. 2012, 70, 87–93. [Google Scholar] [CrossRef]
- Valachovic, P.; Pechova, A.; Mason, T. Towards the industrial production of medicinal tincture by ultrasound assisted extraction. Ultrason. Sonochem. 2001, 8, 111–117. [Google Scholar] [CrossRef]
- Xia, E.Q.; Yu, Y.Y.; Xu, X.R.; Deng, G.F.; Guo, Y.J.; Li, H.B. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Ultrason. Sonochem. 2012, 19, 772–776. [Google Scholar] [CrossRef]
- Ding, L.; Luo, X.B.; Tang, F.; Yuan, J.B.; Guo, M.; Yao, S.Z. Quality control of medicinal herbs Fructus gardeniae, common andrographis herb and their preparations for their active constituents by high-performance liquid chromatography–photodiode array detection-electrospray mass spectrometry. Talanta 2008, 74, 1344–1349. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Wang, Z.T.; Wang, C.H. Comparative pharmacokinetic studies of andrographolide and its metabolite of 14-deoxy-12-hydroxy-andrographolide in rat by ultra-performance liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2013, 27, 931–937. [Google Scholar] [CrossRef]
- Lim, J.C.W.; Chan, T.K.; Ng, D.S.; Sagineedu, S.R.; Stanslas, J.; Wong, W. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef]
- Matsuda, T.; Kuroyanagi, M.; Sugiyama, S.; Umehara, K.; Ueno, A.; Nishi, K. Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull. 1994, 42, 1216. [Google Scholar] [CrossRef]
- Wu, W.; Yan, C.; Li, L.; Liu, Z.; Liu, S. Studies on the flavones using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2004, 1047, 213–220. [Google Scholar]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Dong, H.J.; Zhang, Z.J.; Yu, J.; Liu, Y.; Xu, F.G. Chemical fingerprinting of Andrographis paniculata (Burm. f.) Nees by HPLC and hierarchical clustering analysis. J. Chromatogr. Sci. 2009, 47, 931–935. [Google Scholar] [CrossRef]
- Lin, Q.; Kuang, Y.H.; Huang, L.; Yao, X.H.; Wang, Z.M.; Wang, D.Q.; Li, C.Y. Determination of lactones in andrographis herba and its preparation by quantitative analysis of multi-components by single-marker. Chinese Herb. Med. 2012, 43, 2406–2411. [Google Scholar]
- Sample Availability: Samples of the compounds 6, 8 and 11 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, Y.-X.; Liu, S.-P.; Jin, Z.; Qin, J.-F.; Jiang, Z.-Y. Qualitative and Quantitative Analysis of Andrographis paniculata by Rapid Resolution Liquid Chromatography/Time-of-Flight Mass Spectrometry. Molecules 2013, 18, 12192-12207. https://doi.org/10.3390/molecules181012192
Song Y-X, Liu S-P, Jin Z, Qin J-F, Jiang Z-Y. Qualitative and Quantitative Analysis of Andrographis paniculata by Rapid Resolution Liquid Chromatography/Time-of-Flight Mass Spectrometry. Molecules. 2013; 18(10):12192-12207. https://doi.org/10.3390/molecules181012192
Chicago/Turabian StyleSong, Yong-Xi, Shi-Ping Liu, Zhao Jin, Jian-Fei Qin, and Zhi-Yuan Jiang. 2013. "Qualitative and Quantitative Analysis of Andrographis paniculata by Rapid Resolution Liquid Chromatography/Time-of-Flight Mass Spectrometry" Molecules 18, no. 10: 12192-12207. https://doi.org/10.3390/molecules181012192
APA StyleSong, Y.-X., Liu, S.-P., Jin, Z., Qin, J.-F., & Jiang, Z.-Y. (2013). Qualitative and Quantitative Analysis of Andrographis paniculata by Rapid Resolution Liquid Chromatography/Time-of-Flight Mass Spectrometry. Molecules, 18(10), 12192-12207. https://doi.org/10.3390/molecules181012192




