Metabolite Analysis of Toosendanin by an Ultra-High Performance Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry Technique
Abstract
:1. Introduction
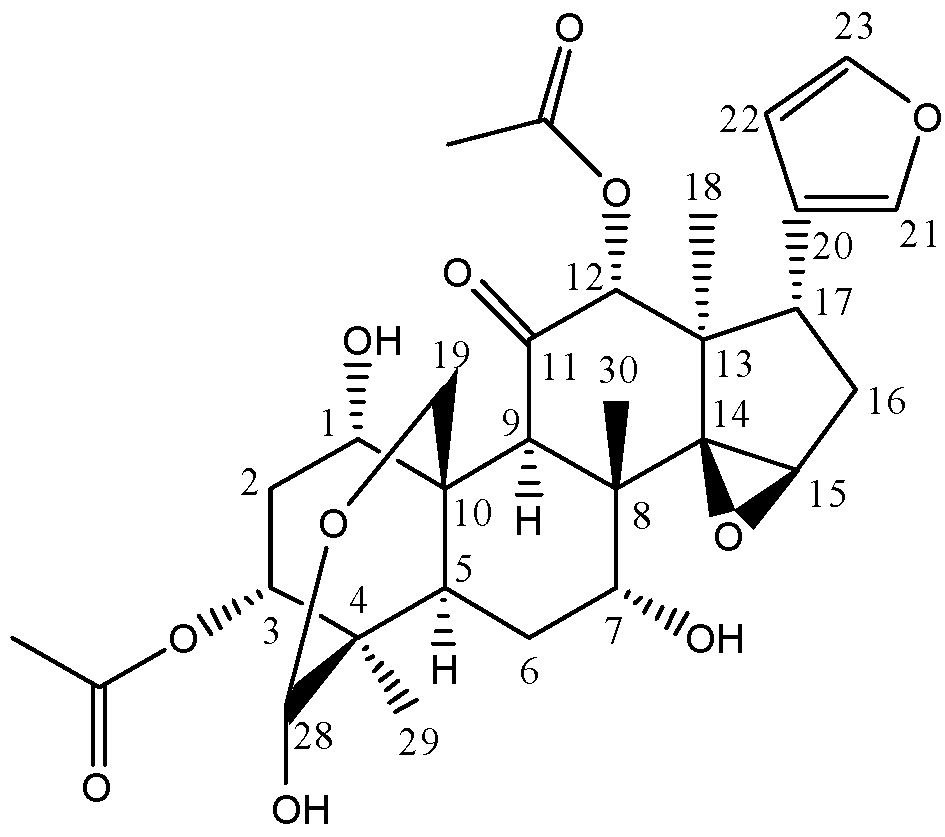
2. Results and Discussion
2.1. Mass Spectrometric Characterization of Toosendanin
2.2. Metabolism of Toosendanin in Vitro by Human Liver Microsomes

2.2.1. Quantification of Toosendanin by UHPLC-QQQ/MS
2.2.2. Identification of Metabolites by UHPLC-Q-TOF/MS
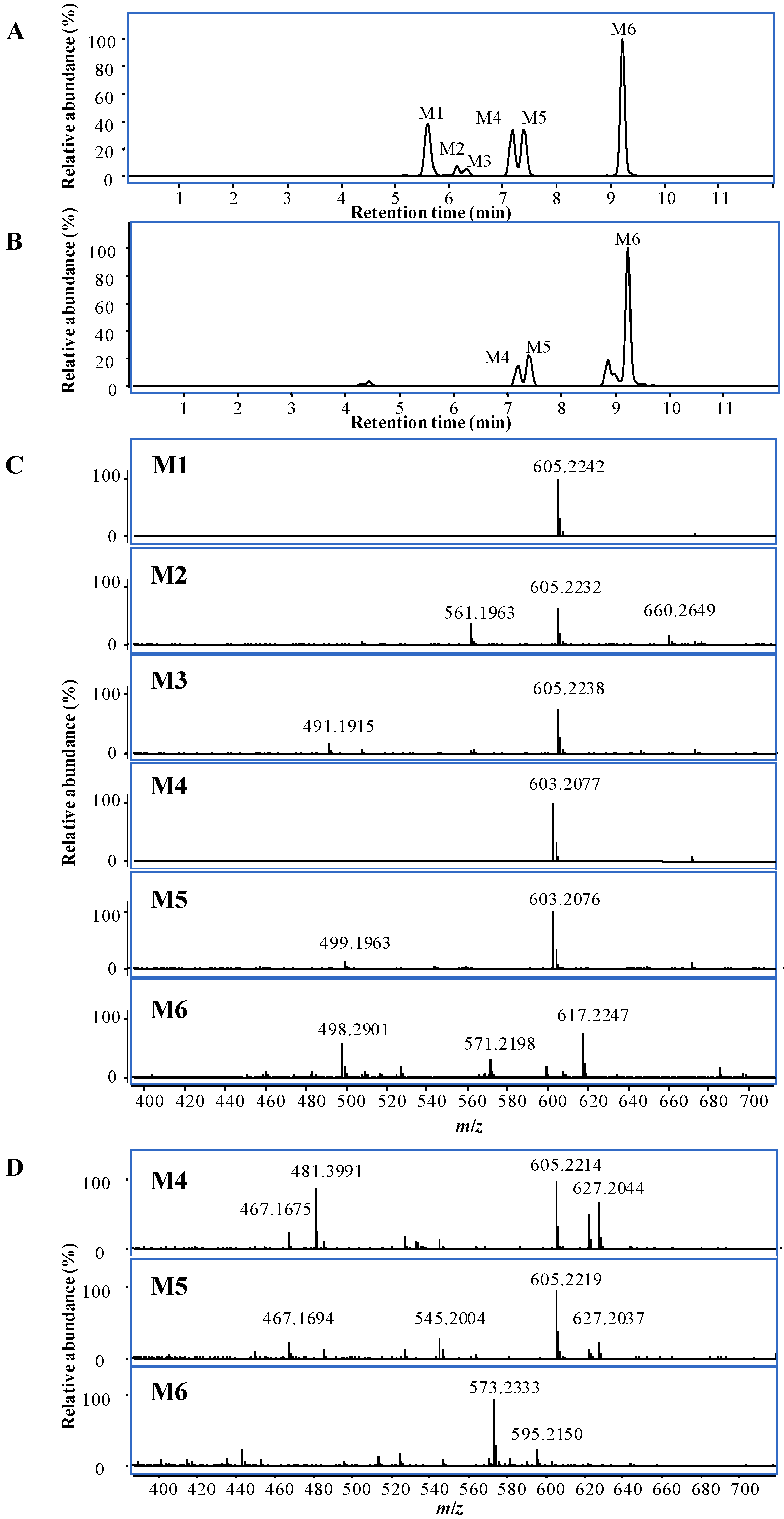
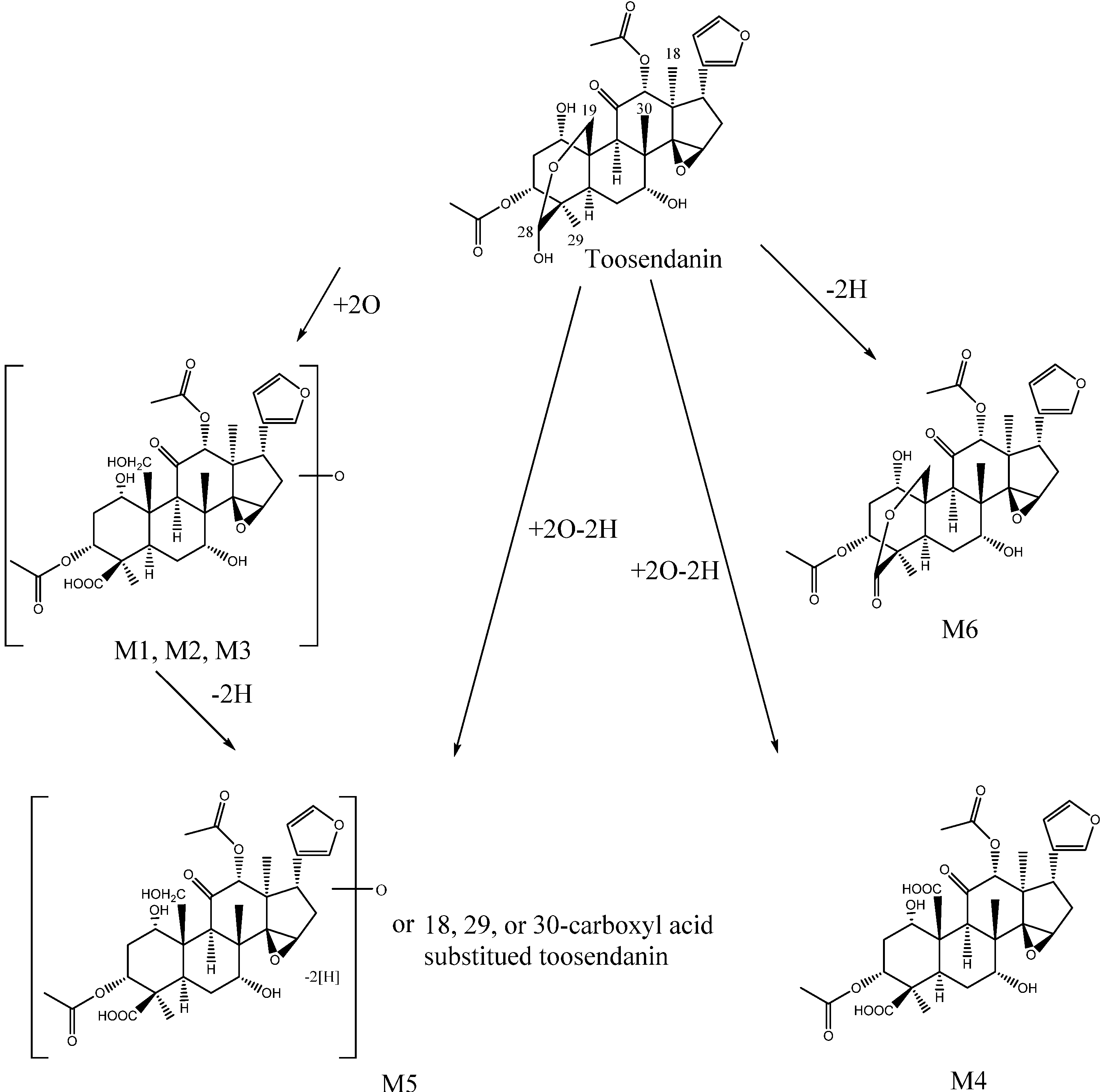
3. Experimental
3.1. Chemicals and Reagents
3.2. Incubation of Toosendanin In Vitro with Human Liver Microsomes
3.3. Quantitative Analysis of Toosendanin by UHPLC-QQQ/MS
3.4. Qualitative Analysis by UHPLC-Q-TOF/MS
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- China Pharmacopoeia Commission, Pharmacopeia of the People’s Republic of China, 2010 ed.; Chemical Industry Press: Beijing, China, 2010.
- Sun, J.; Zhou, H.L. Investigation on clinical application of toosendan fructus. J. Liaoning Univ. Trad. Chin. Med. 2008, 10, 27–28. [Google Scholar]
- Fang, X.F.; Cui, Z.J. The anti-botulism triterpenoid toosendanin elicits calcium increase and exocytosis in rat sensory neurons. Cell. Mol. Neurobiol. 2011, 31, 1151–1162. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.F.; Tang, M.Z.; Shi, Y.L. Growth inhibition and apoptosis-induced effect on human cancer cells of toosendanin, a triterpenoid derivative from chinese traditional medicine. Investigat. New Drugs 2005, 23, 547–553. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Liu, X.; Zhang, L.; Yi, G.; Li, C.; He, X.; Wang, P.; Jiang, H. Toosendanin inhibits hepatocellular carcinoma cells by inducing mitochondria-dependent apoptosis. Planta Med. 2010, 76, 1447–1453. [Google Scholar] [CrossRef]
- Qi, S.Y.; Xiong, Y.H.; Jin, R.M. Time-dose-effect studies on hepatotoxicity of mice induced by fructus toosendan. LiShiZhen Med. Materia Med. Res. 2008, 19, 2694–2696. [Google Scholar]
- Xiong, Y.H.; Qi, S.Y.; Jin, R.M.; Chen, D.X.; Huang, Y.W. Time-dose-effect studies on hepatotoxicity of rats induced by fructus toosendan. Jiangsu J. Tradit. Chin. Med. 2008, 40, 83–85. [Google Scholar]
- Qi, S.Y.; Jin, R.M.; Liu, H.J.; Huang, Y.W. Mechanism studies on hepatotoxicity of rats induced by fructus toosendan. China J. Chin. Materia Med. 2008, 33, 2045–2047. [Google Scholar]
- Zhang, Y.; Qi, X.; Gong, L.; Li, Y.; Liu, L.; Xue, X.; Xiao, Y.; Wu, X.; Ren, J. Roles of reactive oxygen species and map kinases in the primary rat hepatocytes death induced by toosendanin. Toxicology 2008, 249, 62–68. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Wang, Z. Determination of toosendanin in rat plasma by ultra-performance liquid chromatography-electrospray ionization-mass spectrometry and its application in a pharmacokinetic study. Biomed. Chromatogr. 2013, 27, 222–227. [Google Scholar] [CrossRef]
- Xie, C.; Zhong, D.F.; Yu, K.; Chen, X.Y. Recent advances in metabolite identification. Bioanaysis 2012, 4, 937–959. [Google Scholar] [CrossRef]
- Ong, E.S.; Ong, C.N. Qualitative and quantitative analysis of toosendanin in Melia toosendan Sieb. Et Zucc (Meliaceae) with liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 589–598. [Google Scholar] [CrossRef]
- Ramanathan, R.; Comezoglu, S.N.; Humphreys, W.G. Metabolite identification strategies and procedures. In Using Mass Spectrometry for Drug Metabolism Studies, 2nd ed.; Korfmacher, W.A., Ed.; CRC Press: Boca Raton, Florida, USA, 2010; pp. 127–203. [Google Scholar]
- Chen, L.J.; DeRose, E.F.; Burka, L.T. Metabolism of furans in vitro ipomeanine and 4-ipomeanol. Chem. Res. Toxicol. 2006, 19, 1320–1329. [Google Scholar] [CrossRef]
- Peterson, L.A. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem. Res. Toxicol. 2012, 26, 6–25. [Google Scholar] [CrossRef]
- Dalvie, D.K.; Kalgutkar, A.S.; Khojasteh-Bakht, S.C.; Obach, R.S.; O’Donnell, J.P. Biotransformation reactions of five-membered aromatic heterocyclic rings. Chem. Res. Toxicol. 2002, 15, 269–299. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, J.-L.; Leung, E.L.-H.; Zhou, H.; Liu, L.; Li, N. Metabolite Analysis of Toosendanin by an Ultra-High Performance Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry Technique. Molecules 2013, 18, 12144-12153. https://doi.org/10.3390/molecules181012144
Wu J-L, Leung EL-H, Zhou H, Liu L, Li N. Metabolite Analysis of Toosendanin by an Ultra-High Performance Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry Technique. Molecules. 2013; 18(10):12144-12153. https://doi.org/10.3390/molecules181012144
Chicago/Turabian StyleWu, Jian-Lin, Elaine Lai-Han Leung, Hua Zhou, Liang Liu, and Na Li. 2013. "Metabolite Analysis of Toosendanin by an Ultra-High Performance Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry Technique" Molecules 18, no. 10: 12144-12153. https://doi.org/10.3390/molecules181012144




