Synthesis and Promising in Vitro Antiproliferative Activity of Sulfones of a 5-Nitrothiazole Series
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
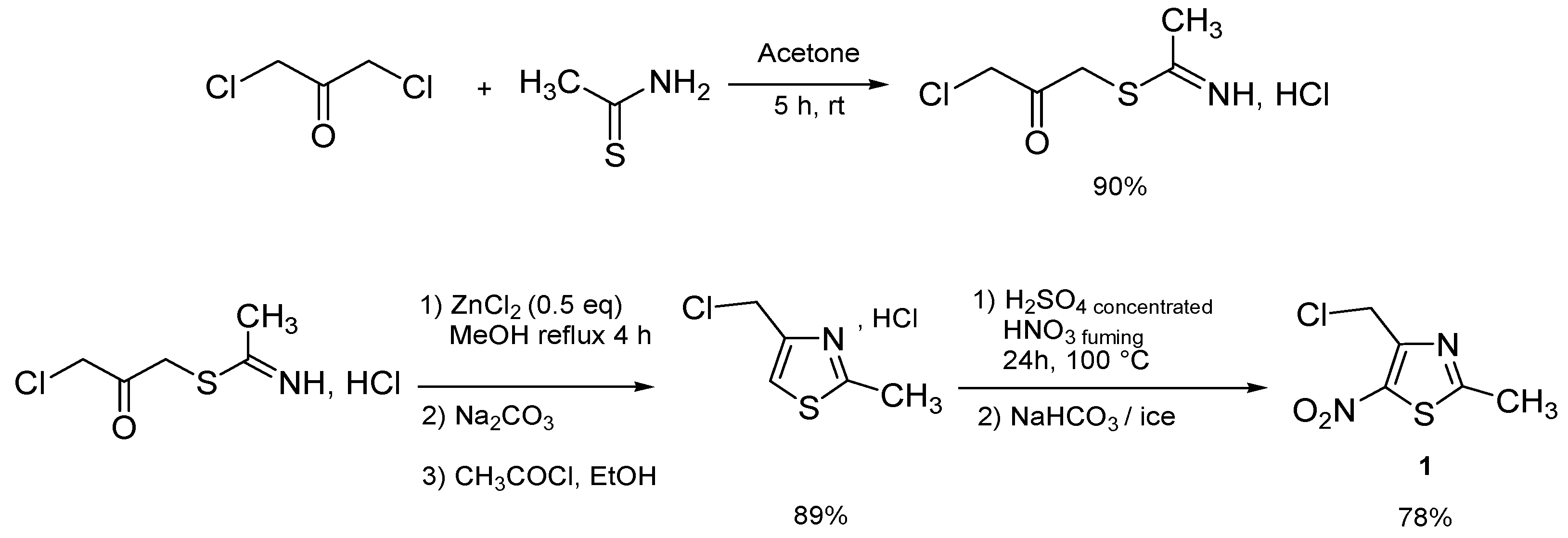
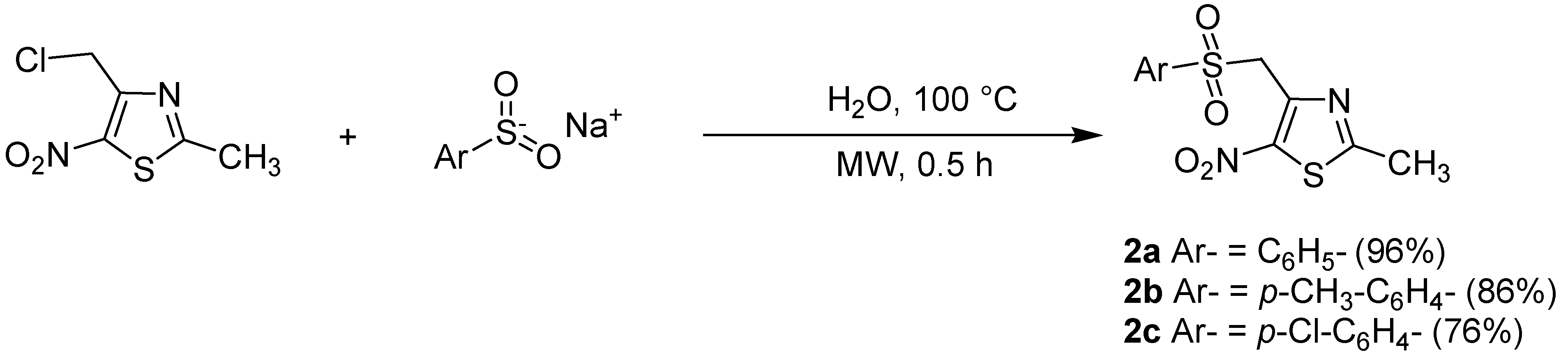
| Entry | Ar- | Product | Product number | Classical heating conditions a | Microwave irradiation conditions b | ||
|---|---|---|---|---|---|---|---|
| Time (h) | Yield (%) | Time (h) | Yield (%) | ||||
| 1 | C6H5- | 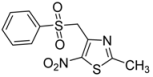 | 2a | 24 | 84 [32] | 0.5 | 96 |
| 2 | p-CH3-C6H4- | 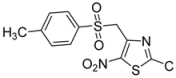 | 2b | 24 | 57 | 0.5 | 86 |
| 3 | p-Cl-C6H4- | 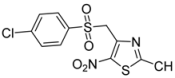 | 2c | 24 | 69 | 0.5 | 76 |
| R- | Product | Product number | Yield (%) |
|---|---|---|---|
| p-Br-C6H4- |  | 2d | 68 |
| p-F-C6H4- | 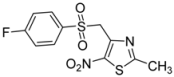 | 2e | 82 |
| m-F-C6H4- | 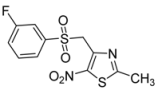 | 2f | 65 |
| m-CF3-C6H4- | 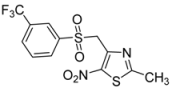 | 2g | 71 |
| p-CH3O-C6H4- |  | 2h | 60 |
| p-C2H5-C6H4- | 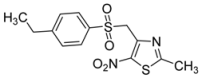 | 2i | 31 |
| CH3- | 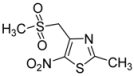 | 2j | 52 |
| 2-bromothiophenyl- |  | 2k | 58 |
| 2-naphthyl- | 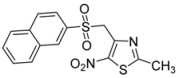 | 2l | 90 |

| R- | Product | Product number | Yield (%) |
|---|---|---|---|
| C6H5- |  | 3a | 81 |
| p-CH3-C6H4- | 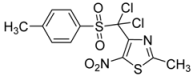 | 3b | 61 |
| -Cl-C6H4- | 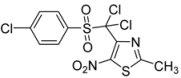 | 3c | 68 |
| p-Br-C6H4- | 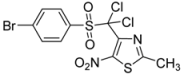 | 3d | 79 |
| p-F-C6H4- | 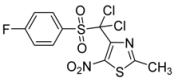 | 3e | 88 |
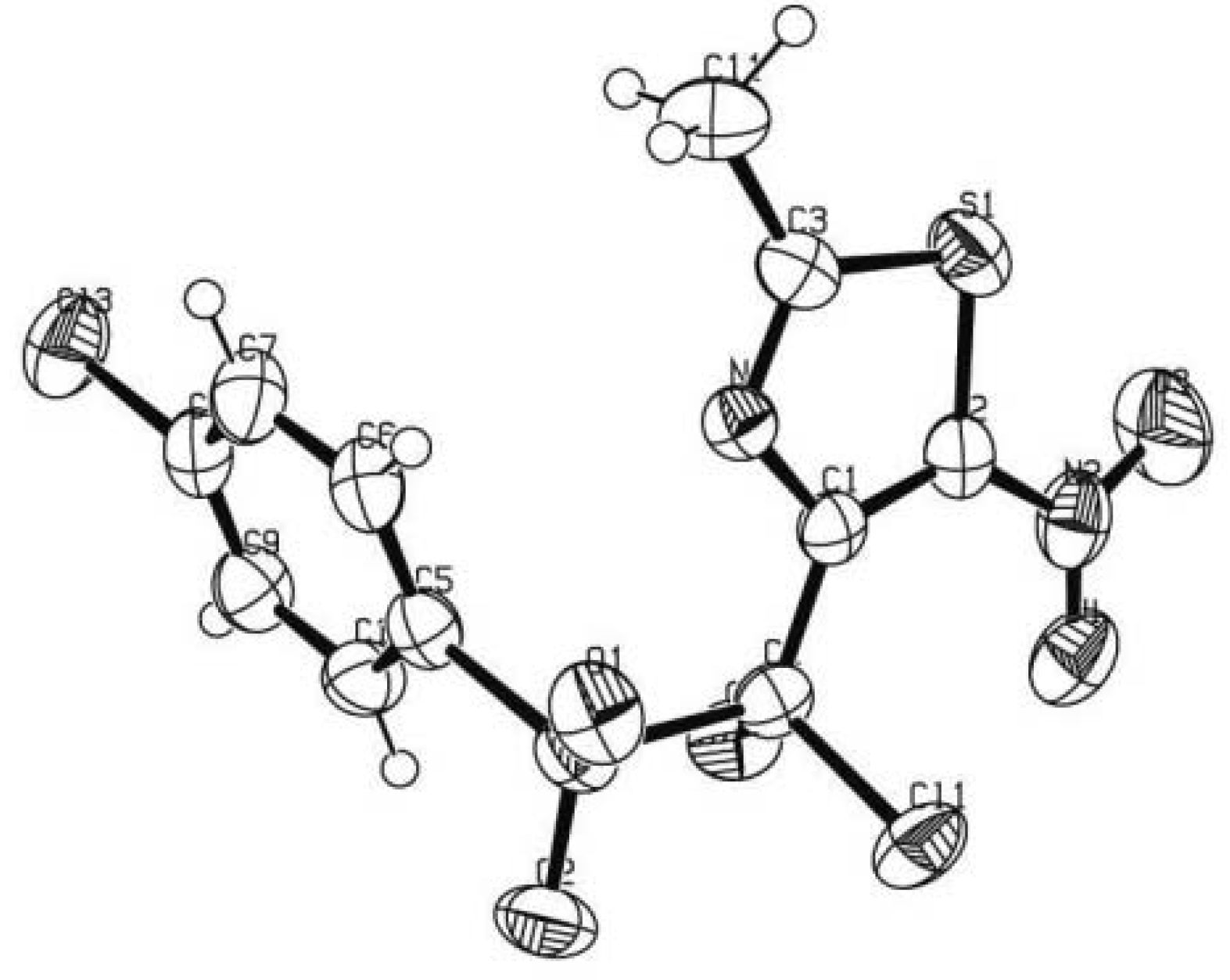
2.2. In Vitro Biological Evaluation
| Product Number | Cancer cell toxicity a (µM) | |
|---|---|---|
| CHO CC50 | HepG2 CC50 | |
| 2a | 322.9 (± 4.66) | 24.6 (± 0.78) |
| 2b | 237.3 (± 5.55) | 7.7 (± 1.42) |
| 2c | >62.5 c | 13.4 (± 1.47) |
| 2d | >500 c | 11.7 (± 2.09) |
| 2e | 229.3 (± 4.02) | 19.3 (± 1.21) |
| 2f | 321.1 (± 3.23) | 23.6 (± 0.58) |
| 2g | 138.6 (± 2.64) | 25.6 (± 2.13) |
| 2h | 136.8 (± 4.26) | 20.6 (± 0.74) |
| 2i | >500 c | 238.9 (± 2.27) |
| 2j | >250 c | >250 c |
| 2k | 47.3 (± 2.28) | 13.8 (± 1.07) |
| 2l | 106.2 (± 4.90) | 8.5 (± 1.52) |
| 3a | 2.5 (± 0.23) | 1.2 (± 0.09) |
| 3b | 1.2 (± 0.11) | 1.0 (± 0.24) |
| 3c | 1.4 (± 0.06) | 1.1 (± 0.17) |
| 3d | 1.3 (± 0.04) | 1.2 (± 0.22) |
| 3e | 1.3 (± 0.04) | 1.2 (± 0.34) |
| Doxorubicin b | 0.6 | 0.2 |
3. Experimental
3.1. General
3.2. General Procedure for the Reaction of Compound 1 and Sodium Arylsulfinates to Synthesize Products 2a to 2c and Using Classical Heating Conditions
3.3. General Procedure for the Reaction of Compound 1 and Sodium Arylsulfinates to Synthesize Products 2a to 2c and Using Microwave Irradiation
3.4. General Procedure for the Reaction of Compound 1 and Variously Substituted Sulfinate Salts to Synthesize Products 2d to 2l and Using Microwave Irradiation
3.5. General Procedure for the Dichlorination of Compounds 2a to 2e to Synthesize Products 3a to 3e Using Microwave Irradiation
3.6. In Vitro Biological Evaluation
4. Conclusions
Acknowledgments
Conflicts of Interest
- Sample Availability: Samples of the compounds 2a to 3e are available from the authors.
References
- Krahn, D.; Ottmann, C.; Kaiser, M. Macrocyclic proteasome inhibitors. Curr. Med. Chem. 2011, 18, 5052–5060. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signalling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Fortin, S.; Wei, L.; Moreau, E.; Lacroix, J.; Côté, M.F.; Petitclerc, E.; Kotra, L.P.; Gaudreault, R.C. Substituted phenyl-4-(2-oxoimidazolin-1-yl)benzenesulfonamides as antimitotics. Antiproliferative, antiangiogenic and antitumoral activity, and quantitative structure-activity relationships. Eur. J. Med. Chem. 2011, 46, 5327–5342. [Google Scholar] [CrossRef]
- Chen, J.; Liu, T.; Wu, R.; Lou, J.; Cao, J.; Dong, X.; Yang, B.; He, Q.; Hu, Y. Design, synthesis, and biological evaluation of novel N-γ-carboline arylsulfonamides as anticancer agents. Bioorg. Med. Chem. 2010, 18, 8478–8484. [Google Scholar]
- Bocca, C.; Bozzo, F.; Bassignana, A.; Miglietta, A. Antiproliferative effects of COX-2 inhibitor celecoxib on human breast cancer cell lines. Mol. Cell. Biochem. 2011, 350, 59–70. [Google Scholar] [CrossRef]
- Park, J.H.; El-Gamal, M.I.; Lee, Y.S.; Oh, C.H. New imidazo[2,1-b]thiazoles derivatives: Synthesis, in vitro anticancer evaluation, and in silico studies. Eur. J. Med. Chem. 2011, 46, 5769–5777. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Zhuang, X.; Luo, J.; Cao, X.; Li, H.; Tu, Z.; Lu, X.; Ren, X.; Ding, K. New thiazole carboxamides as potent inhibitors of Akt kinases. Bioorg. Med. Chem. Lett. 2012, 22, 1208–1212. [Google Scholar]
- Metzger, J.V. Thiazole and Its Derivatives, 1st ed; John Wiley and Sons: New York, NY, USA, 1979; and references therein. [Google Scholar]
- Sykes, R.B.; Cimarusti, C.M.; Bonner, D.P.; Bush, K.; Floyd, D.M.; Georgopapadakou, N.H.; Koster, W.H.; Liu, W.C.; Parker, W.L.; Principe, P.A.; et al. Monocyclic beta-lactam antibiotics produced by bacteria. Nature 1981, 291, 489–491. [Google Scholar]
- Angehrn, P.; Reiner, R. Cephalosporin derivatives and their pharmaceutical preparation. Chem. Abstr. 1983, 98, 22269, Eur. Patent 0,058,250, filed 17 February 1981, issued 25 August 1982. [Google Scholar]
- Sader, H.S.; Johnson, D.M.; Jones, R.N. In vitro activities of the novel cephalosporin LB 11058 against multidrug-resistant Staphylococci and Streptococci. Antimicrob. Agents Chemother. 2004, 48, 53–62. [Google Scholar] [CrossRef]
- Schumacher, H.R., Jr.; Becker, M.A.; Wortmann, R.L.; MacDonald, P.A.; Hunt, B.; Streit, J.; Lademacher, C.; Joseph-Ridge, N. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Care Res. 2008, 59, 1540–1548. [Google Scholar] [CrossRef]
- Barnes, J.; Boutwood, A.; Haines, E.; Lewington, W.; Lister, E.; Haram, B.J. Oral treatment of Trichomonas vaginitis with aminitrozole. Br. Med. J. 1957, 1, 1160–1162. [Google Scholar]
- White, A.C. Jr. Nitazoxanide: A new broad spectrum antiparasitic agent. Expert Rev. Anti-Infect. Ther. 2004, 2, 43–50. [Google Scholar] [CrossRef]
- Gonzalez Cabrera, D.; Douelle, F.; Feng, T.-S.; Nchinda, A.T.; Younis, Y.; White, K.L.; Wu, Q.; Ryan, E.; Burrows, J.N.; Waterson, D.; et al. Novel orally active antimalarial thiazoles. J. Med. Chem. 2011, 54, 7713–7719. [Google Scholar]
- Patai, S.; Rappoport, Z.; Stirling, C. The Chemistry of Sulphones and Sulphoxides, 1st ed; John Wiley and Sons: Chichester, UK, 1988. [Google Scholar]
- Simpkins, N.S. Sulphones in Organic Synthesis, 1st ed; Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Crozet, M.D.; Perfetti, P.; Kaafarani, M.; Crozet, M.P.; Vanelle, P. Rapid syntheses of nitroheterocycles that bear a diethyl methylenemalonate group β to a nitro group. Lett. Org. Chem. 2004, 1, 326–330. [Google Scholar] [CrossRef]
- Vanelle, P.; De Meo, M.P.; Maldonado, J.; Nouguier, R.; Crozet, M.P.; Laget, M.; Dumenil, G. Genotoxicity in oxazolidine derivatives: Influence of the nitro group. Eur. J. Med. Chem. 1990, 25, 241–250. [Google Scholar] [CrossRef]
- Gellis, A.; Kovacic, H.; Boufatah, N.; Vanelle, P. Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur. J. Med. Chem. 2008, 43, 1858–1864. [Google Scholar] [CrossRef]
- Crozet, M.D.; Botta, C.; Gasquet, M.; Curti, C.; Remusat, V.; Hutter, S.; Chapelle, O.; Azas, N.; De Meo, M.; Vanelle, P. Lowering of 5-nitroimidazole’s mutagenicity: Towards optimal antiparasitic pharmacophore. Eur. J. Med. Chem. 2009, 44, 653–659. [Google Scholar]
- Juspin, T.; Laget, M.; Terme, T.; Azas, N.; Vanelle, P. TDAE-assisted synthesis of new imidazo[2,1-b]thiazole derivatives as anti-infectious agents. Eur. J. Med. Chem. 2010, 45, 840–845. [Google Scholar] [CrossRef]
- Bouhlel, A.; Curti, C.; Dumètre, A.; Laget, M.; Crozet, M.D.; Azas, N.; Vanelle, P. Synthesis and evaluation of original amidoximes as antileishmanaial agents. Bioorg. Med. Chem. 2010, 18, 7310–7320. [Google Scholar] [CrossRef]
- Verhaeghe, P.; Dumètre, A.; Castera-Ducros, C.; Hutter, S.; Laget, M.; Fersing, C.; Prieri, M.; Yzombard, J.; Sifredi, F.; Rault, S.; et al. 4-Thiophenoxy-2-trichloromethylquinazolines display in vitro selective antiplasmodial activity against the human malaria parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2011, 21, 6003–6006. [Google Scholar]
- Crozet, M.P.; Archaimbault, G.; Vanelle, P.; Nouguier, R. Réactions SRN1 en série hétérocycliques: IV: Réactivité des sels du diméthyl-2,2 nitro-5 dioxanne-1,3. Tetrahedron Lett. 1985, 26, 5133–5134. [Google Scholar] [CrossRef]
- Zink, L.; Crozet, M.D.; Terme, T.; Vanelle, P. Long distance-SRN1 in nitroimidazole series favored by temperature. Tetrahedron Lett. 2011, 52, 6991–6996. [Google Scholar]
- Crozet, M.D.; Zink, L.; Remusat, V.; Curti, C.; Vanelle, P. Efficient microwave-assisted palladium-catalyzed Suzuki-Miyaura cross-coupling reactions in 5-nitroimidazole series. Synthesis 2009, 3150–3156. [Google Scholar]
- Kabri, Y.; Verhaeghe, P.; Gellis, A.; Vanelle, P. Regioselective Suzuki-Miyaura reaction: Application to the microwave-promoted synthesis of 4,7-diarylquinazolines. Molecules 2010, 15, 2949–2961. [Google Scholar] [CrossRef]
- Crozet, M.D.; Castera-Ducros, C.; Vanelle, P. An efficient microwave-assisted Suzuki cross-coupling reaction of imidazo[1,2-a]pyridines in aqueous medium. Tetrahedron Lett. 2006, 47, 7061–7065. [Google Scholar] [CrossRef]
- Cohen, A.; Crozet, M.D.; Rathelot, P.; Vanelle, P. An efficient aqueous microwave-assisted Suzuki-Miyaura cross-coupling reaction in the thiazole series. Green Chem. 2009, 11, 1736–1742. [Google Scholar] [CrossRef]
- Hooper, F.E.; Johnson, T.B. The polymerization of 2-methyl-4-chloromethylthiazole. J. Am. Chem. Soc. 1934, 56, 470–471. [Google Scholar] [CrossRef]
- Gellis, A.; Vanelle, P.; Kaafarani, M.; Benakli, K.; Crozet, M.P. Synthèse et réactions SRN1 en série 5-nitrothiazole. Tetrahedron 1997, 53, 5471–5484. [Google Scholar] [CrossRef]
- Crozet, M.P.; Giraud, L.; Sabuco, J.-F.; Vanelle, P.; Barreau, M. SRN1 reactions of a tetrasubstituted-1,4-benzoquinone. Tetrahedron Lett. 1991, 32, 4125–4128. [Google Scholar] [CrossRef]
- Crozet, M.P.; Gellis, A.; Pasquier, C.; Vanelle, P.; Aune, J.-P. Electron transfer reactivity in 5-nitrouracile series. Tetrahedron Lett. 1995, 36, 525–528. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal microwave effects revisited: On the importance of internal temperature monitoring and agitation in microwave chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Aqueous microwave chemistry: A clean and green synthetic tool for rapiddrug discovery. Chem. Soc. Rev. 2008, 37, 1546–1557. [Google Scholar]
- Grieco, P.A. Organic Synthesis in Water, 1st ed; Blackie Academic and Professional: London, UK, 1998. [Google Scholar]
- Li, C.-J.; Chan, T.H. Comprehensive Organic Reactions in Aqueous Media, 2nd ed; John Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lindström, U.M. Organic Reactions in Water, 1st ed; Blackwell: Ames, IA, USA, 2007. [Google Scholar]
- De Borggraeve, W.M.; Appukkattan, P.; Azzam, R.; Dehaen, W.; Compernolle, F.; Van der Eycken, E.; Hoornaert, G. Synthesis of novel functionalized symmetric bi-2(1H)-pyrazinones. Synlett 2005, 777–780. [Google Scholar]
- Gellis, A.; Boufatah, N.; Vanelle, P. Rapid microwave-promoted synthesis of new sulfonylmethylbenzothiazoles in water. Green Chem. 2006, 8, 483–487. [Google Scholar] [CrossRef]
- Jia, C.-S.; Dong, Y.-W.; Tu, S.-J.; Wang, G.-W. Microwave-assisted solvent-free synthesis of substituted 2-quinolones. Tetrahedron 2007, 63, 892–897. [Google Scholar] [CrossRef]
- Kabri, Y.; Gellis, A.; Vanelle, P. Synthesis of original 2-substituted 4-arylquinazolines (III) by microwave-irradiated Suzuki-Miyaura cross-coupling reactions. Eur. J. Org. Chem. 2009, 24, 4059–4066. [Google Scholar]
- Curti, C.; Laget, M.; Ortiz Carle, A.; Gellis, A.; Vanelle, P. Rapid synthesis of sulfone derivatives as potential anti-infectious agents. Eur. J. Med. Chem. 2007, 42, 880–884. [Google Scholar] [CrossRef]
- Field, L.; Clark, R.D. Methyl p-tolyl sulfone. Org. Synth. 1958, 38, 62–65. [Google Scholar]
- Antane, S.; Bernotas, R.; Li, Y.; McDevitt, R.; Yan, Y. Chloromethyl sulfones from sulfonyl chlorides via a one-pot procedure. Synth. Commun. 2004, 34, 2443–2449. [Google Scholar] [CrossRef]
- Liu, L.K.; Chi, Y.; Jen, K. Copper-catalyzed additions of sulfonyl iodides to simple and cyclic alkenes. J. Org. Chem. 1980, 45, 406–410. [Google Scholar] [CrossRef]
- Kidwai, M.; Kohli, S.; Kumar, P. Rapid side-chain chlorination of heterocyclic compounds using focused microwave irradiation. J. Chem. Res. 1998, 586–587. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rodriguez-Antona, C.; Donato, M.T.; Boobis, A.; Edwards, R.J.; Watts, P.S.; Castell, J.V.; Gómez-Lechón, M.J. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: Molecular mechanisms that determine lower expression in cultured cells. Xenobiotica 2002, 32, 505–520. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cohen, A.; Crozet, M.D.; Rathelot, P.; Azas, N.; Vanelle, P. Synthesis and Promising in Vitro Antiproliferative Activity of Sulfones of a 5-Nitrothiazole Series. Molecules 2013, 18, 97-113. https://doi.org/10.3390/molecules18010097
Cohen A, Crozet MD, Rathelot P, Azas N, Vanelle P. Synthesis and Promising in Vitro Antiproliferative Activity of Sulfones of a 5-Nitrothiazole Series. Molecules. 2013; 18(1):97-113. https://doi.org/10.3390/molecules18010097
Chicago/Turabian StyleCohen, Anita, Maxime D. Crozet, Pascal Rathelot, Nadine Azas, and Patrice Vanelle. 2013. "Synthesis and Promising in Vitro Antiproliferative Activity of Sulfones of a 5-Nitrothiazole Series" Molecules 18, no. 1: 97-113. https://doi.org/10.3390/molecules18010097





