Synthesis and Biological Evaluation of 3-Aryl-quinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives as Hypoxic Selective Anti-tumor Agents
Abstract
:1. Introduction
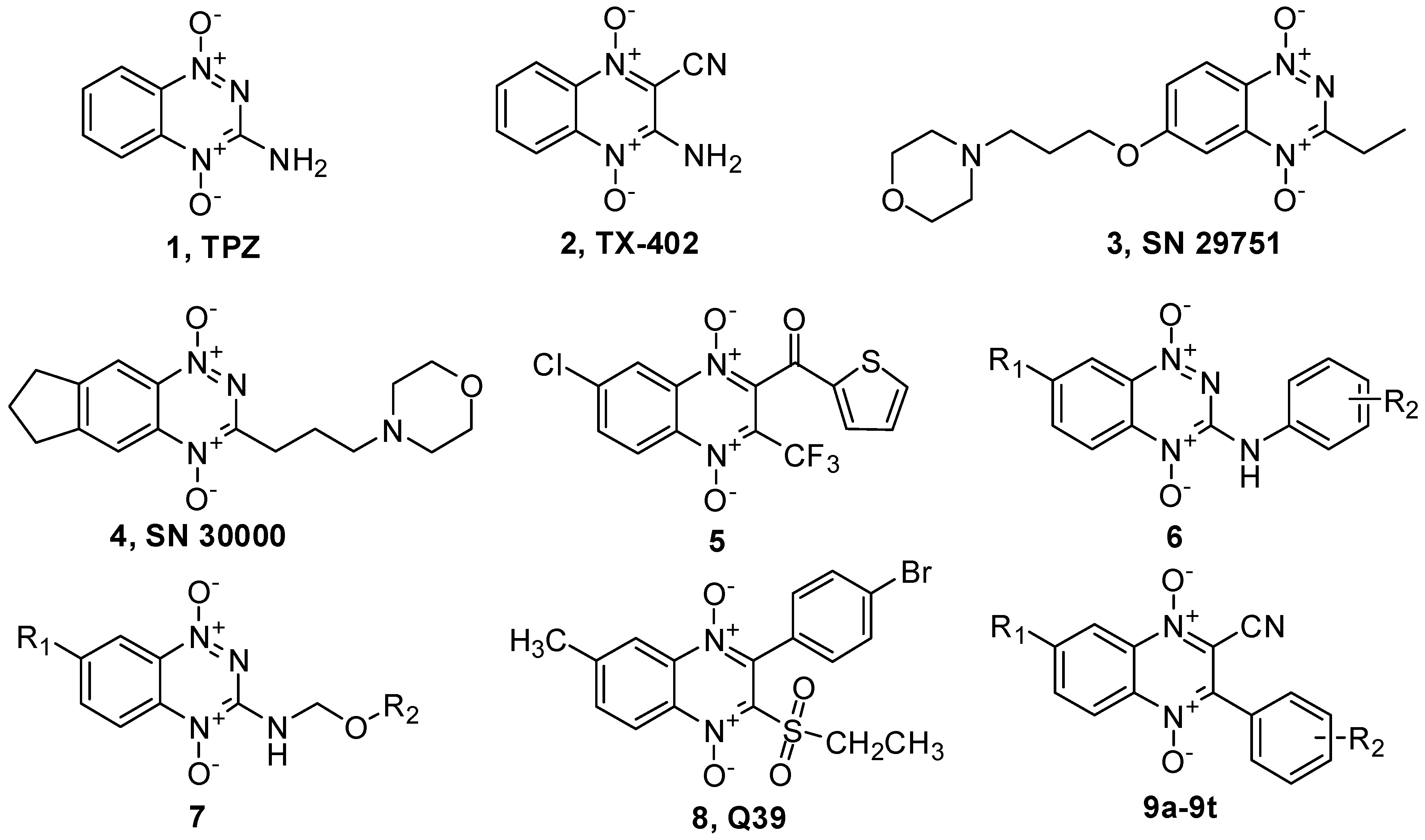
2. Results and Discussion
2.1. Chemistry
2.2. Pharmacology
2.2.1. In Vitro Cytotoxic Activity
| Comp. | R1 | R2 | Cytotoxicity(IC50, μM) and HCR | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMMC-7721 | K562 | KB | A549 | PC3 | |||||||||||||
| H a | N b | HCR c | H | N | HCR | H | N | HCR | H | N | HCR | H | N | HCR | |||
| TX-402 | - | - | >50 | >50 | - | 13.1 | >50 | - | 0.98 | 8.85 | 9.03 | >50 | >50 | - | 5.87 | >50 | - |
| TPZ | - | - | 4.75 | 32.79 | 6.90 | 1.81 | 19.41 | 10.72 | 18.71 | 6.29 | 0.34 | 1.93 | 7.43 | 3.85 | 1.17 | 20.97 | 17.92 |
| 9a | H | H | 1.58 | 15.7 | 9.94 | 17.53 | 45.4 | 2.59 | 1.53 | 16.46 | 10.76 | 8.08 | 52 | 6.44 | 25 | 24.6 | 0.98 |
| 9b | H | 3-CH3 | 5.07 | 100 | 19.72 | 17.7 | 15.7 | 0.89 | 7.9 | 14.75 | 1.87 | 21 | 26.3 | 1.25 | 18.2 | 7.56 | 0.42 |
| 9c | H | 3-Cl | 1.09 | 5 | 4.59 | 1.03 | 11.32 | 10.99 | 0.76 | 4.59 | 6.04 | 10.61 | 46.71 | 4.40 | 3 | 15.03 | 5.01 |
| 9d | H | 4-Br | 1.82 | 32.9 | 18.08 | 19.58 | 47.1 | 2.41 | 5.08 | 13.61 | 2.68 | 11.2 | 19.8 | 1.77 | 16.4 | >50 | >3.05 |
| 9e | H | 4-NO2 | 1.43 | 8.28 | 5.79 | 4.01 | 3.98 | 0.99 | 2.83 | 12 | 4.24 | 8.02 | 42.31 | 5.28 | 10.36 | >50 | >4.83 |
| 9f | CH3 | H | 0.63 | 100 | 158.73 | 2.04 | 22.6 | 11.08 | 4.54 | 34.9 | 7.69 | 9.38 | 12.5 | 1.33 | 3.8 | 24.2 | 6.37 |
| 9g | CH3 | 3-CH3 | 2.98 | 2.97 | 1.00 | 0.64 | 3.16 | 4.94 | 0.72 | 10.65 | 14.79 | 21.72 | >50 | >2.30 | 6.21 | >50 | >8.05 |
| 9h | CH3 | 3-Cl | 0.76 | 10.34 | 13.61 | 0.92 | 7.54 | 8.20 | 0.53 | 2.19 | 4.13 | 4.91 | 11.13 | 2.27 | 2.25 | 12.55 | 5.57 |
| 9i | CH3 | 4-Br | 0.93 | 6.18 | 6.65 | 1.07 | 4.32 | 4.04 | 0.17 | 5.96 | 35.06 | 36.15 | 32.46 | 0.90 | 5.54 | 20.26 | 3.66 |
| 9j | CH3 | 4-NO2 | 1.6 | 43 | 26.88 | 6.59 | 13.3 | 2.02 | 5.12 | 8.71 | 1.70 | 17 | 25 | 1.47 | 28.3 | 39 | 1.38 |
| 9k | OCH3 | H | 1.16 | 78 | 67.24 | 7.98 | 16.8 | 2.11 | 3.97 | 66.72 | 16.81 | 6.31 | 4.46 | 0.71 | 6.76 | 43.5 | 6.43 |
| 9l | OCH3 | 3-CH3 | 1.7 | 6.23 | 3.66 | 0.76 | 13.53 | 17.80 | 1.16 | 6.23 | 5.37 | 15.3 | >50 | >3.27 | 4.41 | >50 | >11.34 |
| 9m | OCH3 | 3-Cl | 0.9 | 4.83 | 5.37 | 2.02 | 6.76 | 3.35 | 1.42 | 4.87 | 3.43 | 8.75 | >50 | >5.71 | 2.25 | 17.55 | 7.80 |
| 9n | OCH3 | 4-Br | 0.12 | 13.84 | 115.33 | 1.37 | 3.34 | 2.44 | 4.03 | 6.63 | 1.65 | 11.32 | 28.43 | 2.51 | 8.4 | 28.61 | 3.41 |
| 9o | OCH3 | 4-NO2 | 1.62 | 3.92 | 2.42 | 4.49 | 9.23 | 2.06 | 5.41 | 3.54 | 0.65 | 6.17 | 6.53 | 1.06 | 18.7 | 25.2 | 1.35 |
| 9p | Cl | H | 0.46 | 4.27 | 9.28 | 2.66 | 9.39 | 3.53 | 0.33 | 3.25 | 9.85 | 5.75 | 30.84 | 5.36 | 3.89 | 27.19 | 6.99 |
| 9q | Cl | 3-CH3 | 0.37 | 1.73 | 4.68 | 0.79 | 0.87 | 1.10 | 0.62 | 11.85 | 19.11 | 12.3 | 18.5 | 1.50 | 9.93 | >50 | >5.04 |
| 9r | Cl | 3-Cl | 0.8 | 1.73 | 2.16 | 1.73 | 4.62 | 2.67 | 0.51 | 1.92 | 3.76 | 11.77 | 11.74 | 1.00 | 3.06 | 11.98 | 3.92 |
| 9s | Cl | 4-Br | 2.08 | 23.7 | 11.39 | 16.55 | 38.5 | 2.33 | 3.63 | 17.82 | 4.91 | 33.59 | 4.1 | 0.12 | 50.9 | 13.3 | 0.26 |
| 9t | Cl | 4-NO2 | 3.4 | 23.6 | 6.94 | 1.67 | 1.48 | 0.89 | 4.92 | 2.38 | 0.48 | 5.72 | 5.4 | 0.94 | 16.1 | 13.8 | 0.86 |
| Cell line | IC50 (mM) | HCR c | |
|---|---|---|---|
| H a | N b | ||
| Human hepatoma BEL-7402 | 2.23 | 14.7 | 6.60 |
| Human hepatoma HepG2 | 1.76 | 13.1 | 7.44 |
| Human promyelocytic leukemia HL-60 | 3.16 | 4.80 | 1.52 |
| Human lung cancer NCI-H460 | 2.64 | 5.96 | 2.26 |
| Human colon cancer HCT-116 | 1.71 | 4.92 | 2.88 |
| Human neuroblastoma CHP126 | 0.31 | 5.51 | 17.8 |
2.2.2. Mechanism Studies
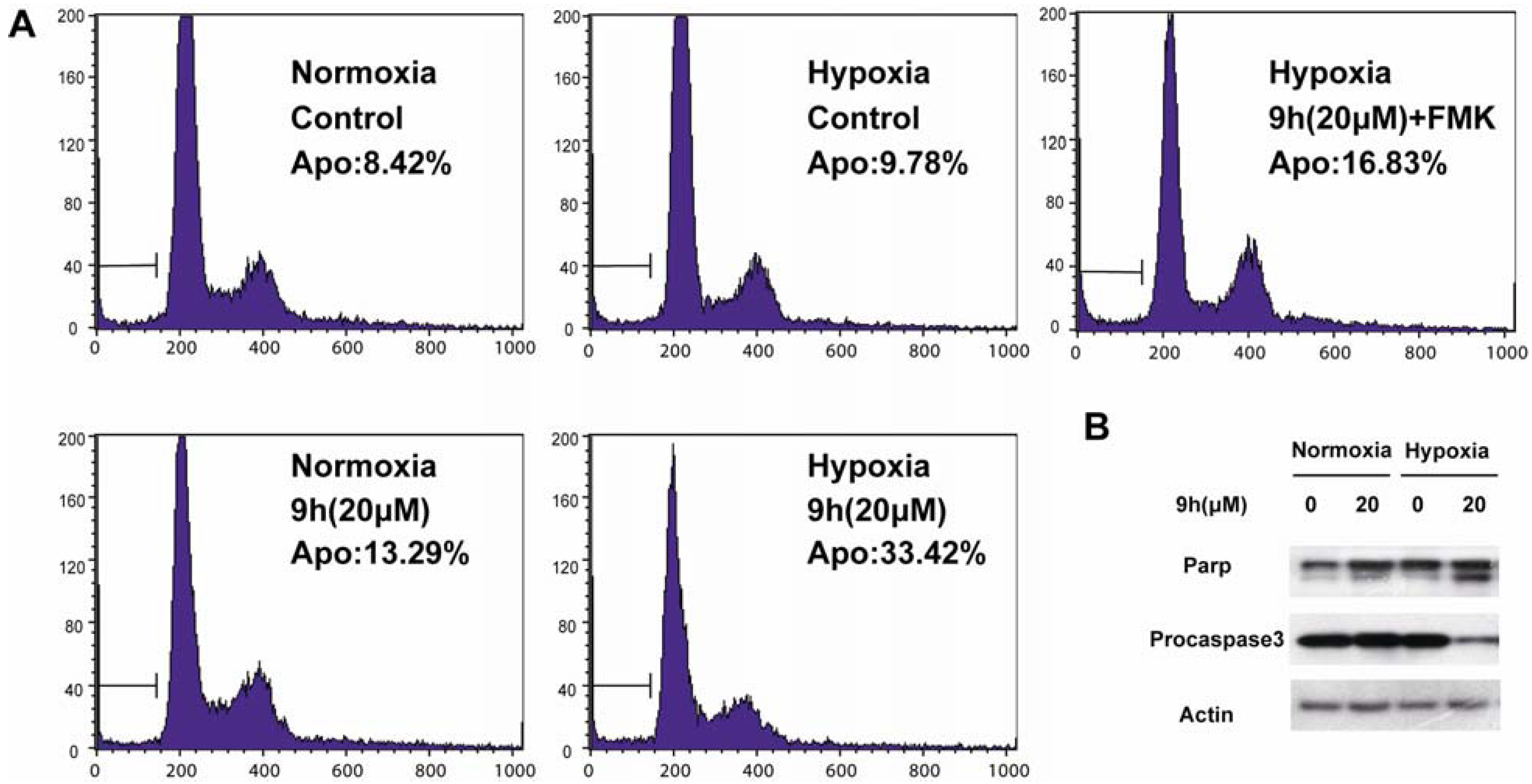
3. Experimental
3.1. General
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Benzoylacetonitriles 11a–e
3.2.2. General Procedure for the Synthesis of 3-Aryl-2-quinoxalinecarbonitrile-1,4-di-N-oxide Derivatives 9a–t
3.3. Pharmacology
3.3.1. Cytotoxicity Assay [25]
3.3.2. Flow Cytometry Analysis [25]
3.3.3. Western Blot Analysis [25]
4. Conclusions
Acknowledgements
References
- Martin, B.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Wardman, P. The importance of radiation chemistry to radiation and free radical biology (The 2008 silvanus thompson memorial lecture). Br. J. Radiol. 2009, 82, 89–104. [Google Scholar]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Rischin, D.; Peters, L.; Fisher, R.; Macann, A.; Denham, J.; Poulsen, M.; Jackson, M.; Kenny, L.; Penniment, M.; Corry, J.; et al. Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: A randomized phase II trial of the trans-tasman radiation oncology group (TROG 98.02). J. Clin. Oncol. 2005, 23, 79–87. [Google Scholar]
- Rischin, D.; Peters, L.; ÓSullivan, B.O.; Giralt, J.; Fisher, R.; Yuen, K.; Trotti, A.; Bernier, J.; Bourhis, J.; Ringash, J.; et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): A phase III trial of the trans-tasman radiation oncology group. J. Clin. Oncol. 2010, 28, 2989–2996. [Google Scholar]
- Monge, A.; Martinez-Crespo, F.J.; Lopez, A.; Palop, J.A.; Narro, S.; Senador, V.; Marin, A.; Sainz, Y.; Gonzalez, M. Hypoxia-selective agents derived from 2-Quinoxalinecarbonitrile 1,4-Di-N-oxides. 2. J. Med. Chem. 1995, 38, 4488–4494. [Google Scholar] [CrossRef]
- Hay, M.P.; Hicks, K.O.; Pchalek, P.; Lee, H.H.; Blaser, A.; Pruijn, F.B.; Anderson, R.F.; Shinde, S.S.; Wilson, W.R.; Denny, W.A. Tricyclic [1,2,4]triazine 1,4-dioxides as hypoxia selective cytotoxins. J. Med. Chem. 2008, 51, 6853–6865. [Google Scholar] [CrossRef]
- Solano, B.; Junnotula, V.; Marín, A.; Villar, R.; Burguete, A.; Vicente, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Dutta, S.; et al. Synthesis and biological evaluation of new 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives and their reduced analogues. J. Med. Chem. 2007, 50, 5485–5492. [Google Scholar]
- Magda, M.F.I.; Kamelia, M.A.; Eman, N.; Dalia, H.S.; Yousry, A.A. New quinoxaline 1, 4-di-N-oxides: Anticancer and hypoxia-selective therapeutic agents. Eur. J. Med. Chem. 2010, 45, 2733–2738. [Google Scholar]
- Hicks, K.O.; Siim, B.G.; Jaiswal, J.K.; Pruijn, F.B.; Fraser, A.M.; Patel, R.; Hogg, A.; Liyanage, S.; Dorie, M.J.; Brown, M.; et al. Pharmacokinetic/Pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin. Cancer Res. 2010, 16, 4946–4957. [Google Scholar]
- Jiang, F.Q.; Yang, B.; Fan, L.L.; He, Q.J.; Hu, Y.Z. Synthesis and hypoxic-cytotoxic activity of some 3-amino-1,2,4-benzotriazine-1,4-dioxide derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 4209–4213. [Google Scholar]
- Xia, Q.; Zhang, L.; Zhang, J.; Sheng, R.; Yang, B.; He, Q.J.; Hu, Y.Z. Synthesis, hypoxia-selective cytotoxicity of new 3-amino-1,2,4-benzotriazine-1,4-dioxide derivatives. Euro. J. Med. Chem. 2011, 46, 919–926. [Google Scholar]
- Jiang, F.Q.; Weng, Q.J.; Sheng, R.; Xia, Q.; He, Q.J.; Yang, B.; Hu, Y.Z. Synthesis, structure and hypoxic cytotoxicity of 3-amino-1,2,4-benzotriazine-1,4-dioxide derivatives. Arch. Der. Pharm. 2007, 340, 258–263. [Google Scholar] [CrossRef]
- Sheng, R.; Xu, Y.; Weng, Q. J.; Xia, Q.; He, Q.J.; Yang, B.; Hu, Y.Z. Synthesis and cytotoxic activity of 3-phenyl-2-thio-quinoxaline1,4-dioxide derivatives in hypoxia and in normoxia. Drug. Discov. Ther. 2007, 1, 119–123. [Google Scholar]
- Weng, Q.J.; Wang, D.D.; Guo, P.; Fang, L.; Hu, Y.Z. Q39, a novel synthetic quinoxaline 1,4-di-N-oxide compound with anti-cancer activity in hypoxia. Eur. J. Pharmacol. 2008, 581, 262–269. [Google Scholar] [CrossRef]
- Weng, Q.J.; Zhang, J.; Cao, J.; Xia, Q.; Wang, D.D.; Hu, Y.Z.; Sheng, R.; Wu, H.H.; Zhu, D.F.; Zhu, H.; et al. Q39, a quinoxaline 1,4-di-N-oxide derivative, inhibits hypoxia-inducible factor-1α expression and the Akt/mTOR/4E-BP1 signaling pathway in human hepatoma cells. Invest New Drug. 2011, 29, 1177–1187. [Google Scholar]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A.; Maurel, S.; Deharo, E.; Jullian, V.; Sauvain, M. Synthesis and antimalarial activity of new 3-arylquinoxaline-2-carbonitrile derivatives. Arzneim. Forsch. 2005, 55, 754–761. [Google Scholar]
- Vicente, E.; Duchowicz, P.R.; Benitez, D.; Castro, E.A.; Cerecetto, H.; Gonzalez, M.; Monge, A. Anti-T. cruzi activities and QSAR studies of 3-arylquinoxaline-2-carbonitrile di-N-oxides. Bioorg. Med. Chem. Lett. 2010, 20, 4831–4835. [Google Scholar]
- Vicente, E.; Lima, L.M.; Bongard, E.; Charnaud, S.; Villar, R.; Solano, B.; Burguete, A.; Perez-Silanes, S.; Aldana, I.; Vivas, L.; Monge, A. Synthesis and structure-activity relationship of 3-phenylquinoxaline 1,4-di-N-oxide derivatives as antimalarial agents. Eur. J. Med. Chem. 2008, 43, 1903–1910. [Google Scholar] [CrossRef]
- Puterová, Z.; Andicsová, A.; Végh, D. Synthesis of π-conjugated thiophenes starting from substituted 3-oxopropanenitriles via gewald reaction. Tetrahedron 2008, 64, 11262–11269. [Google Scholar]
- Dornow, A.; Kuhlcke, I.; Baxmann, F. Über einige derivate der benzoylessigsäure. Chem. Ber. 1949, 82, 254–257. [Google Scholar] [CrossRef]
- Alon, H.; Lena, L.; Michal, W.; Iris, O.G.; Abraham, N.; Amnon, H. De Novo parallel design, synthesis and evaluation of inhibitors against the reverse transcriptase of human immunodeficiency virus type-1 and drug-resistant variants. J. Med. Chem. 2007, 50, 2370–2383. [Google Scholar]
- Ridge, D.N.; Hanifin, J.W.; Harten, L.A.; Johnson, B.D.; Menschik, J.; Nicolau, G.; Sloboda, A.E.; Watts, D.E. Potential antiarthritic agents. 2. Benzoylacetonitriles and .beta.-aminocinnamonitriles. J. Med. Chem. 1979, 22, 1385–1389. [Google Scholar] [CrossRef]
- Gaughran, R.J.; Picard, J.P.; Kaufman, J.V.R. Contribution to the chemistry of benzfuroxan and benzfurazan derivatives. J. Am. Chem. Soc. 1954, 76, 2233–2236. [Google Scholar] [CrossRef]
- Yang, B.; Patrick, R.C. Tirapazamine cytotoxicity for neuroblastoma is p53 Dependent. Clin. Cancer. Res. 2005, 11, 2774–2780. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, Y.; Xia, Q.; Shangguan, S.; Liu, X.; Hu, Y.; Sheng, R. Synthesis and Biological Evaluation of 3-Aryl-quinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives as Hypoxic Selective Anti-tumor Agents. Molecules 2012, 17, 9683-9696. https://doi.org/10.3390/molecules17089683
Hu Y, Xia Q, Shangguan S, Liu X, Hu Y, Sheng R. Synthesis and Biological Evaluation of 3-Aryl-quinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives as Hypoxic Selective Anti-tumor Agents. Molecules. 2012; 17(8):9683-9696. https://doi.org/10.3390/molecules17089683
Chicago/Turabian StyleHu, Yunzhen, Qing Xia, Shihao Shangguan, Xiaowen Liu, Yongzhou Hu, and Rong Sheng. 2012. "Synthesis and Biological Evaluation of 3-Aryl-quinoxaline-2-carbonitrile 1,4-Di-N-oxide Derivatives as Hypoxic Selective Anti-tumor Agents" Molecules 17, no. 8: 9683-9696. https://doi.org/10.3390/molecules17089683




