N-Substituted 5-Chloro-6-phenylpyridazin-3(2H)-ones: Synthesis, Insecticidal Activity Against Plutella xylostella (L.) and SAR Study
Abstract
:1. Introduction
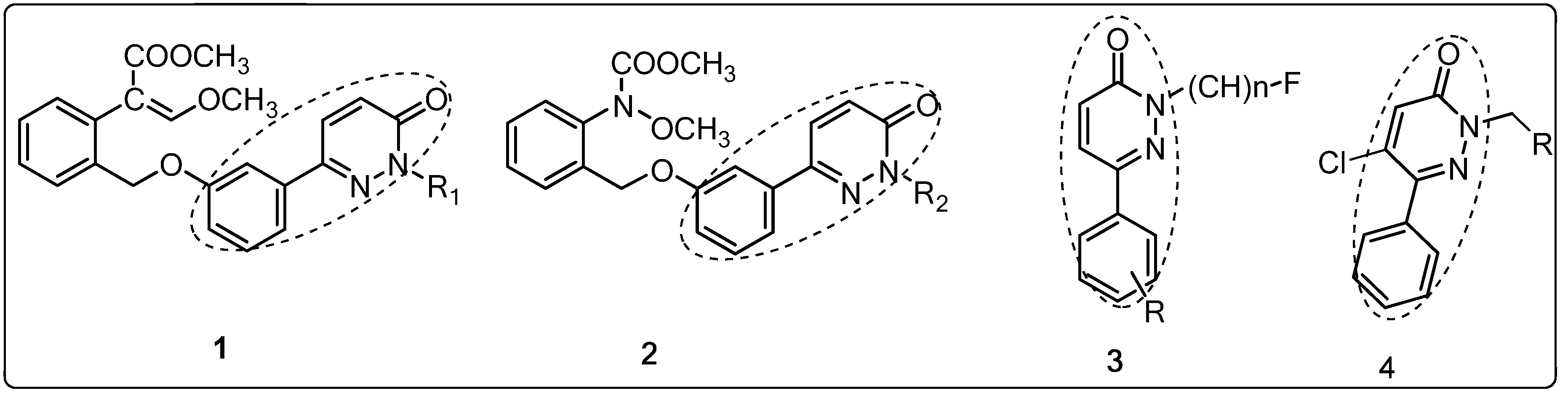
2. Results and Discussion
2.1. Chemistry
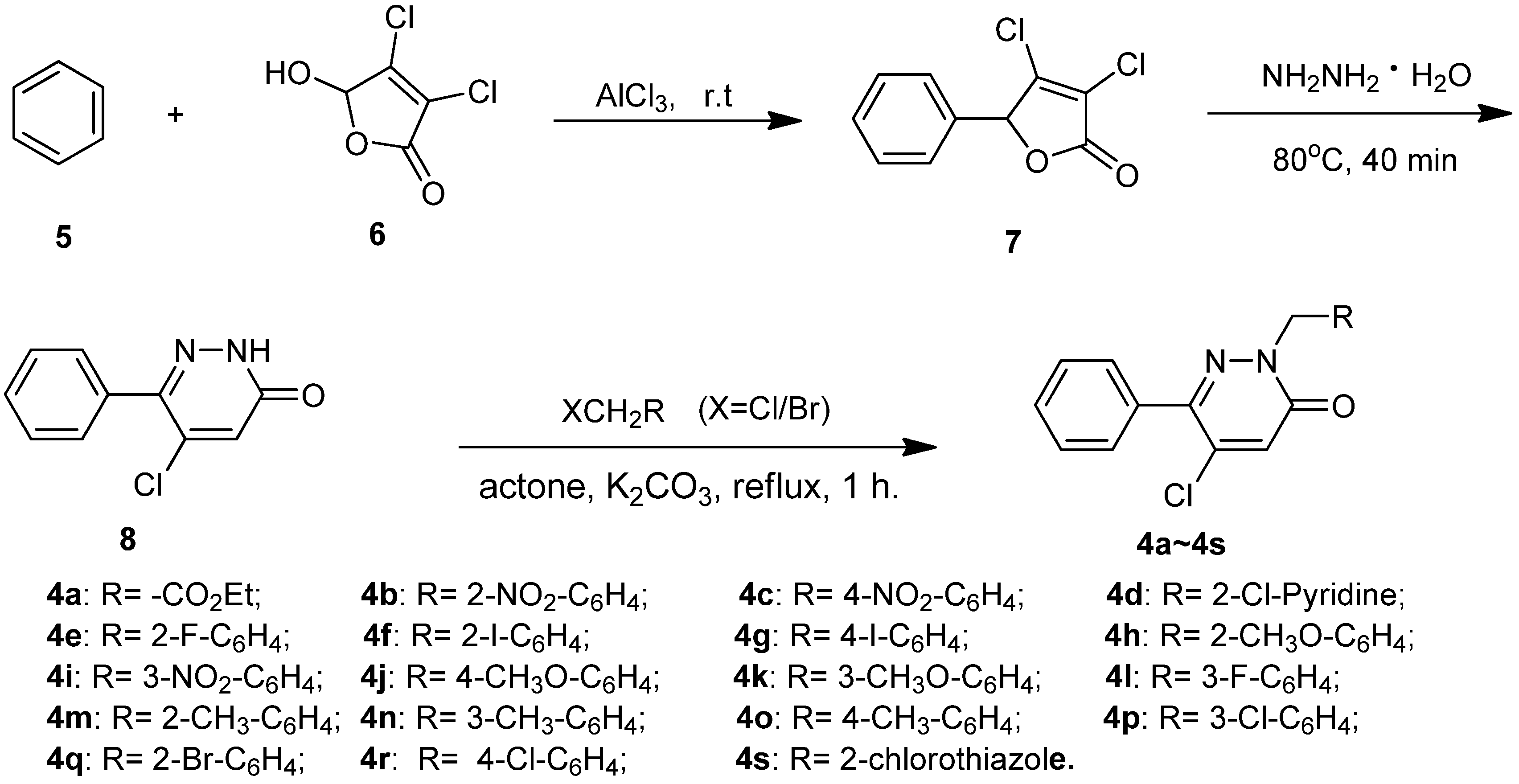
2.2. Insecticidal Activity
2.3. Structure-Activity Relationship (SAR) Study
| Comp. | Insecticidal activity (%) at a concentration of (mg/L) | ||
|---|---|---|---|
| 100 | 50 | 25 | |
| 4a | 20 | / | / |
| 4b | 100 | 97 | 21 |
| 4c | 25 | 0 | / |
| 4d | 93 | 76 | 20 |
| 4e | 13 | / | / |
| 4f | 50 | 13 | 0 |
| 4g | 45 | 0 | / |
| 4h | 97 | 70 | 20 |
| 4i | 10 | 0 | / |
| 4j | 45 | 13 | 0 |
| 4k | 60 | 30 | 13 |
| 4l | 62 | 33 | 0 |
| 4m | 20 | 0 | / |
| 4n | 15 | / | / |
| 4o | 14 | / | / |
| 4p | 21 | 0 | / |
| 4q | 43 | 10 | / |
| 4r | 31 | 0 | / |
| 4s | 84 | 60 | 15 |
| Blank control | 0 | 0 | 0 |
| Chlorpyrifos | 100 | 97 | 67 |
| Avermectin | 100 | 100 | 100 |
3. Experimental
3.1. Chemistry
3.2. Insecticidal Bioassays
4. Conclusions
Acknowledgements
References
- Arora, R.; Battu, G.S.; Bath, D.S. Management of insect pests of coliflower with biopesticides. Indian J. Ecol. 2000, 27, 156–162. [Google Scholar]
- Talekar, N.S.; Shelton, A.M. Biology, ecology and management of the diamondback moth. Ann. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Shelton, A.M.; Tang, J.D.; Roush, R.T.; Metz, T.D.; Earle, E.D. Field tests on managing resistance to Bt-engineered plants. Nat. Biotechnol. 2000, 18, 339–342. [Google Scholar]
- Andrews, K.L.; Sanchez, R.J.; Cave, R.D. Management of diamondback moth in Central America. In Diamondback moth Moth Management, Proceedings of the Second International Workshop, Tainan, Taiwan, 10-14 December 1990; pp. 487–497.
- Cheng, E.Y. Problems of control of insecticide-resistant Plutella xylostella. Pestic. Sci. 1988, 23, 177–188. [Google Scholar] [CrossRef]
- Perez, C.J.; Alvarado, P.; Miranda, F.; Narváez, C.; Hernández, L.; Vanegas, H.; Hruska, A.; Shelton, A.M. Assessment of insecticide resistance in five insect pests attacking field and vegetable crops in Nicaragua. J. Econ. Entomol. 2000, 93, 1779–1787. [Google Scholar] [CrossRef]
- Kasnar, B.; Wise, D.S.; Kucera, L.S.; Drach, J.C.; Townsend, L.B. Synthesis of 2′,3′-dideoxy- and 3′-azido-2′,3′-dideoxypyridazine nucleosides as potential antiviral agents. Nucleos. Nucleot. Nucl. 1994, 13, 459–479. [Google Scholar] [CrossRef]
- Zou, X.J.; Jin, G.Y.; Yang, Z. Synthesis of hydrazides of 1-aryl-1,4-dihydro-6-methyl-4- pyridazinone and their antiviral activity against TMV. Chin. J. Appl. Chem. 2001, 18, 599–601. [Google Scholar]
- Li, R.D.; Zhai, X.; Zhao, Y.F.; Yu, S.; Gong, P. Synthesis and anti-tumor activities of a novel series of tricyclic 1-anilino-5H-pyridazino[4,5-b]indoles. Arch. Pharm. 2007, 340, 424–428. [Google Scholar]
- Hiroshi, M.; Akio, M. Pyridazine compound and use thereof. EP Patent 1,767,529, 28 March 2007. [Google Scholar]
- Hiroshi, M.; Akio, M. Pyridazine compound and use thereof. WO Patent 2,006,001,175, 5 January 2006. [Google Scholar]
- Shinichiro, S.; Shinichiro, M.A.; Akio, M. Pyridazine compound and bactericide containing the same. JP Patent 2,007,182,430, 19 July 2007. [Google Scholar]
- Hoshino, K.; Koike, Y. Halopyridazines as insecticides. JP Patent 2,005,263,694, 29 September 2005. [Google Scholar]
- Ishimukai, M.; Matsusaka, M. Insecticides comprising dihalopyridazines. JP Patent 2,007,077,140, 29 March 2007. [Google Scholar]
- Yang, H.Z.; Xu, H.; Hu, X.H.; Zou, X.M.; Liu, B.; Zhu, Y.Q.; Hu, F.Z. Method for preparation of 3-substituted aminopyridazine derivatives with herbicidal activity. CN Patent 101,058,563, 24 October 2007. [Google Scholar]
- Hu, F.Z.; Zhang, G.F.; Zhu, Y.Q.; Zou, X.M.; Liu, B.; Yang, H.Z. Synthesis and biological activity of 3-aryloxy-6-(3,5-dimethyl-1H-pyrazol-1-yl)-pyridazine. Chin. J. Org. Chem. 2007, 27, 758–762. [Google Scholar]
- Zou, X.M.; Yang, H.Z.; Xu, H.; Liu, B.; Hu, X.H.; Zhu, Y.Q.; Hu, F.Z. Preparation of 4-(trifluoromethylphenyl)pyridazine derivatives as herbicides. CN Patent 1,962,642, 16 May 2007. [Google Scholar]
- Wu, J.; Song, B.A.; Hu, D.Y.; Yang, S. Research advance in insecticidal and acaricidal activities of pyridazine derivatives. Agrochemicals 2011, 50, 630–634. [Google Scholar]
- Ross, R.; Shaber, S.H.; Szapacs, E.M. Dihydropyridazinones and pyridazinones and use as fungicides and insecticides. EP Patent 0738716, 23 October 1996. [Google Scholar]
- Ross, R.; Shaber, S.H.; Szapacs, E.M. Dihydropyridazinones and pyridazinones and use as fungicides and insecticides. US Patent 5,635,494, 3 June 1997. [Google Scholar]
- Lai, H.K.; Paul, T.M.D. Pesticidal phenylpyridazinone derivatives. US Patent 6063781, 16 May 2000. [Google Scholar]
- Wu, J.; Song, B.A.; Chen, H.J.; Bhadury, P.; Hu, D.Y. Synthesis and Antifungal Activity of 5–Chloro-6-Phenylpyridazin-3(2H)-one Derivatives. Molecules 2009, 14, 3676–3687. [Google Scholar] [CrossRef]
- Drury, K. New methods in the chemistry of pyridazones. Angew. Chem. Intern. Eng. Ed. 1965, 4, 292–300. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.L.; Xiong, L.X.; Wang, M.Z.; Li, Y.Q.; Li, Z.M. Synthesis and insecticidal activities of novel anthranilic diamides containing modified N-pyridyl pyrazoles. J. Agric. Food Chem. 2010, 58, 12327–12336. [Google Scholar]
- Wang, Y.J.; Ou, X.M.; Pei, H.; Lin, X.M.; Yu, K. Toxicities of novel insecticide chlorfenpyr against several insects in lab. Agrochem. Res. Appl. 2006, 10, 20–23. [Google Scholar]
- Zhao, Q.Q.; Li, Y.Q.; Xiong, L.X.; Wang, Q.M. Design, synthesis and insecticidal activity of novel phenylpyrazoles containing a 2,2,2-trichloro-1-alkoxyethyl moiety. J. Agric. Food Chem. 2010, 58, 4992–4998. [Google Scholar]
- Sample Availability: Samples of all the intermediates and the title compounds 4a-s are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, J.; Kang, S.; Yuan, Q.; Luo, L.; Ma, J.; Shi, Q.; Yang, S. N-Substituted 5-Chloro-6-phenylpyridazin-3(2H)-ones: Synthesis, Insecticidal Activity Against Plutella xylostella (L.) and SAR Study. Molecules 2012, 17, 9413-9420. https://doi.org/10.3390/molecules17089413
Wu J, Kang S, Yuan Q, Luo L, Ma J, Shi Q, Yang S. N-Substituted 5-Chloro-6-phenylpyridazin-3(2H)-ones: Synthesis, Insecticidal Activity Against Plutella xylostella (L.) and SAR Study. Molecules. 2012; 17(8):9413-9420. https://doi.org/10.3390/molecules17089413
Chicago/Turabian StyleWu, Jian, Shenghong Kang, Qinkun Yuan, Lijun Luo, Juan Ma, Qingcai Shi, and Song Yang. 2012. "N-Substituted 5-Chloro-6-phenylpyridazin-3(2H)-ones: Synthesis, Insecticidal Activity Against Plutella xylostella (L.) and SAR Study" Molecules 17, no. 8: 9413-9420. https://doi.org/10.3390/molecules17089413




