Improvement in the Water Retention Characteristics of Sandy Loam Soil Using a Newly Synthesized Poly(acrylamide-co-acrylic Acid)/AlZnFe2O4 Superabsorbent Hydrogel Nanocomposite Material
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of SHNC

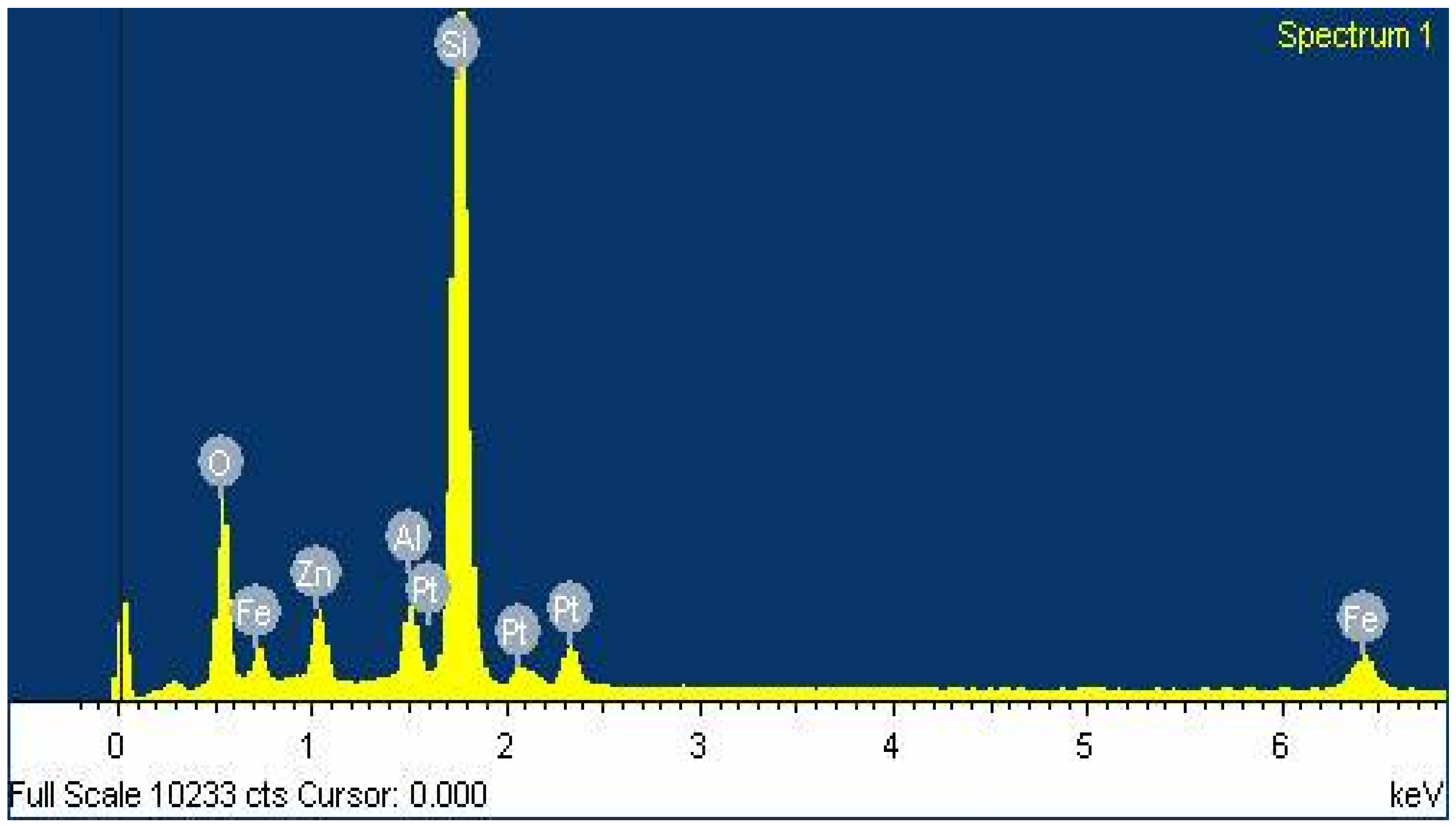

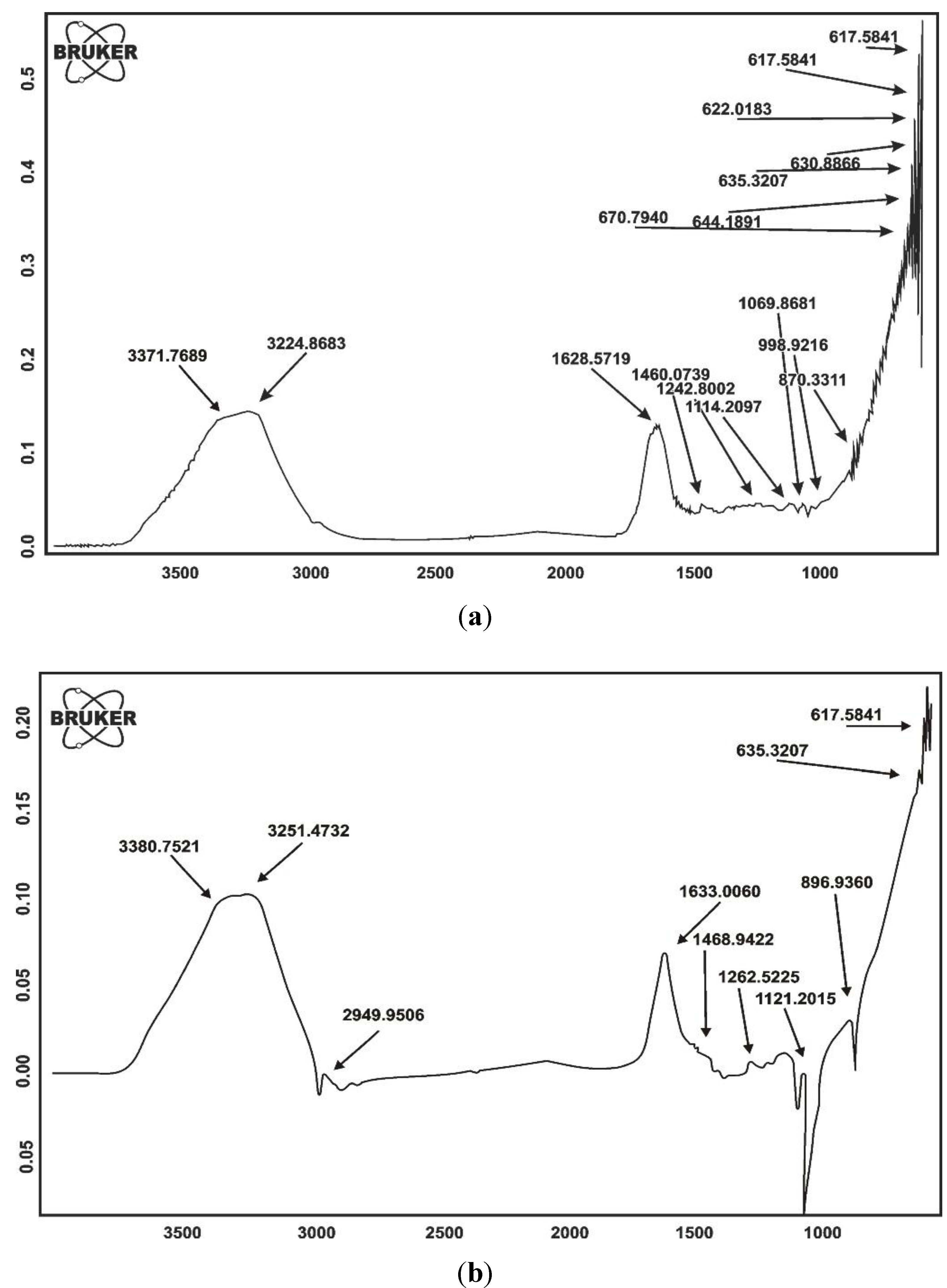
2.1.1. FTIR Spectroscopy
2.2. Water Absorption by SHNC

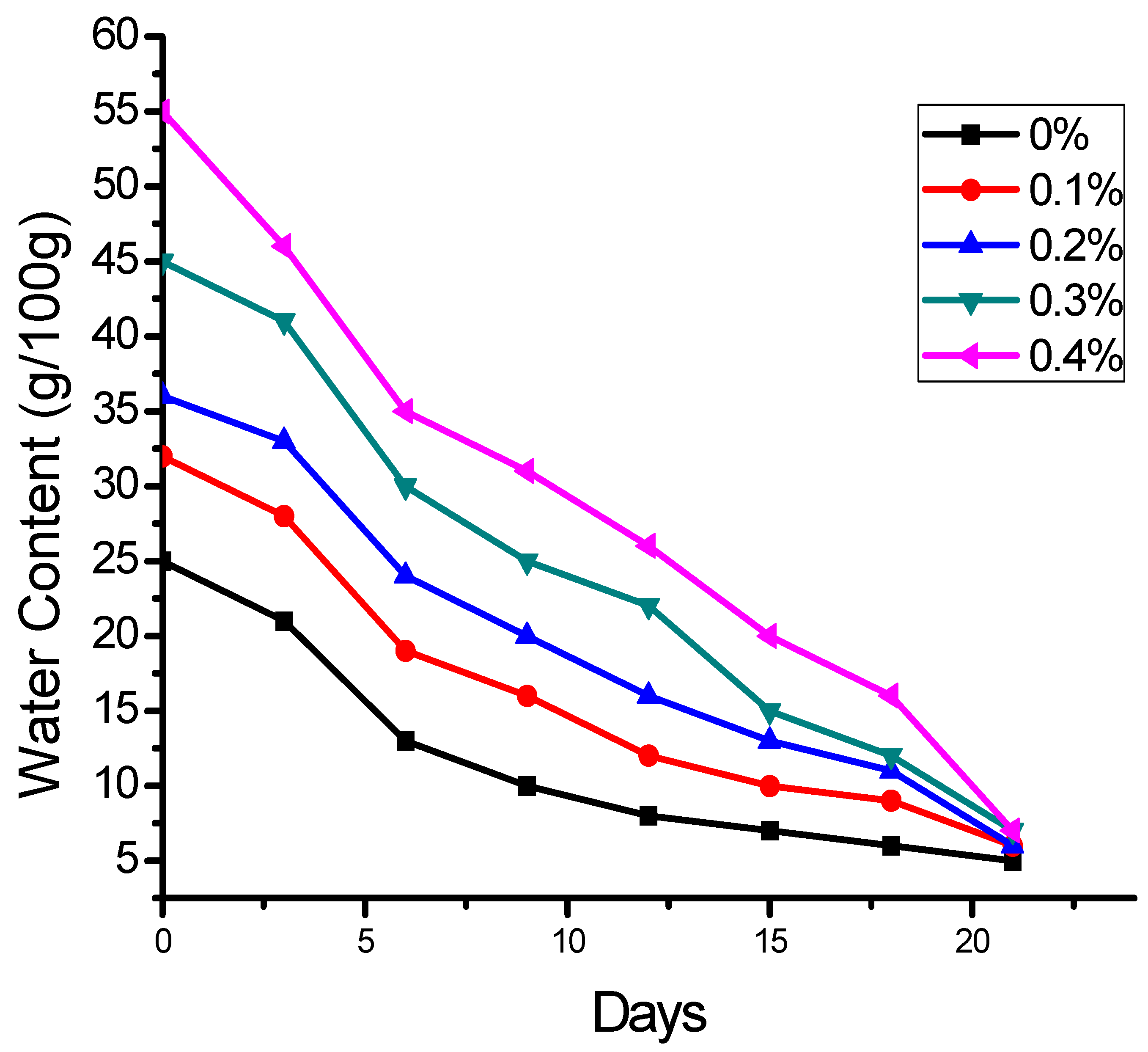
2.3. Effect of SHNC on Moisture Retention in Soil
2.4. Effect of SHNC on Soil pH and Electrical Conductivity (EC)
2.5. Effect on Soil Bulk Density and Porosity
| Hydration | SHNC conc.(%) | Soil pH | Electrical Conductivity (EC) dS/m | Porosity % | Hydraulic Conductivity mm/h | Bulk Density g/cm3 |
|---|---|---|---|---|---|---|
| 0 | 7.40 ± 0.14 | 1.73 ± 0.21 | 33.50 ± 1.73 | 74.92 ± 2.50 | 1.46 ± 0.10 | |
| 0.1 | 7.31 ± 0.27 | 1.83 ± 0.15 | 35.35 ± 1.92 | 62.40 ± 2.24 | 1.41 ± 0.05 | |
| 1st | 0.2 | 7.22 ± 0.12 | 1.92 ± 0.23 | 37.17 ± 2.12 | 47.32 ± 1.65 | 1.40 ± 0.14 |
| 0.3 | 7.12 ± 0.14 | 2.18 ± 0.16 | 41.60 ± 1.82 | 38.25 ± 1.64 | 1.35 ± 0.06 | |
| 0.4 | 7.04 ± 0.21 | 2.73 ± 0.35 | 44.21 ± 2.30 | 27.33 ± 1.28 | 1.27 ± 0.05 | |
| 0 | 7.40 ± 0.12 | 1.73 ± 0.11 | 33.30 ± 1.13 | 74.92 ± 2.40 | 1.46 ± 0.15 | |
| 0.1 | 7.35 ± 0.21 | 1.83 ± 0.12 | 35.14 ± 1.72 | 61.40 ± 2.23 | 1.42 ± 0.09 | |
| 2nd | 0.2 | 7.29 ± 0.11 | 1.91 ± 0.20 | 37.12 ± 2.12 | 44.32 ± 1.55 | 1.40 ± 0.12 |
| 0.3 | 7.22 ± 0.10 | 2.15 ± 0.16 | 40.50 ± 1.22 | 40.25 ± 1.34 | 1.38 ± 0.07 | |
| 0.4 | 7.17 ± 0.19 | 2.47 ± 0.32 | 42.21 ± 2.35 | 37.33 ± 1.18 | 1.32 ± 0.12 | |
| 0 | 7.40 ± 0.11 | 1.73 ± 0.21 | 33.54 ± 1.23 | 74.92 ± 2.30 | 1.46 ± 0.11 | |
| 0.1 | 7.36 ± 0.20 | 1.77 ± 0.19 | 34.45 ± 1.22 | 69.40 ± 2.14 | 1.45 ± 0.10 | |
| 3rd | 0.2 | 7.32 ± 0.11 | 1.82 ± 0.21 | 36.37 ± 2.11 | 59.32 ± 1.45 | 1.42 ± 0.11 |
| 0.3 | 7.31 ± 0.12 | 2.08 ± 0.12 | 38.20 ± 1.42 | 49.25 ± 1.34 | 1.39 ± 0.26 | |
| 0.4 | 7.24 ± 0.20 | 2.33 ± 0.34 | 40.11 ± 2.20 | 40.33 ± 1.21 | 1.37 ± 0.19 |
2.6. Effect of SHNC on Hydraulic Conductivity
2.7. Effect of SHNC on Seed Germination and Seedling Growth of Wheat
| SHNC Level (%) | Seed Germination (%) | Shoot Length (cm) | Shoot Fresh Weight (mg) | Shoot Dry Weight (mg) |
|---|---|---|---|---|
| 0 | 80.2 ± 1.73 a | 16.24 ± 1.91 b | 88.75 ± 2.45 c | 18.11 ± 1.91 b |
| 0.1 | 91.5 ±1.82 ab | 20.76 ± 1.82 ab | 108.55 ± 3.25 b | 20.45 ± 1.82 b |
| 0.2 | 92.0 ± 1.90 a | 22.61 ± 2.05 ab | 118.35 ± 3.62 a | 24.50 ± 1.91 a |
| 0.3 | 92.4 ± 2.12 ab | 24.36 ± 2.15 a | 133.72 ± 4.50 a | 27.20 ± 2.10 a |
| 0.4 | 96.1 ± 2.30 ab | 25.80 ± 2.23 a | 140.00 ±5.24 ab | 32.42 ±2.31ab |
2.8. Effect of SHNC on Permanent Wilting Point
2.9. General Discussion
3. Experimental
3.1. Materials
3.1.1. Soils Used in the Experiment
3.1.2. Chemicals
3.2. Methods
3.2.1. Synthesis of Nano-Sized AlZnFe2O4
- 1. Al2(SO4)3·18 H2O 32.4 g/100 mL Solution A
- 2. ZnSO4·H2O 5.4 g/100 mL Solution B
- 3. FeCl3·6H2O 20.0 g/100 mL Solution C
- 4. NaOH 22.0 g/200 mL Solution D
3.2.2. Syntheses of Poly(AA-co-AAm)/AlZnFe2O4 Superabsorbent Hydrogel Nanocomposite
3.2.3. Physical Characterization
3.2.4. Measurements of the Water Absorbency

3.2.5. Reswelling Capability
3.2.6. Measurement of the Water-Retention Properties in Sandy Loam Soil
3.2.7. Measurement of Soil pH and EC
3.2.8. Measurement of Bulk Density and Porosity

3.2.9. Measurement of Hydraulic Conductivity
3.2.10. Evaluation of Seed germination and Seedling Growth in Amended Soil
3.2.11. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Zhang, J.; Chen, H.; Li, P.; Wang, A. Study on superabsorbent composite, 14. Preparation of poly(acrylic acid)/organo-attapulgite composite hydrogels and swelling behaviors in aqueous electrolyte solution. Macromol. Mat. Eng 2006, 291, 1529–1538. [Google Scholar] [CrossRef]
- Akhter, J.; Mahmood, K.; Malik, K.A.; Mardan, A.; Ahmad, M.; Iqbal, M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar]
- Buchholz, F.L.; Graham, T. Polyether-Modified Poly (acrylic acid): Synthesis and Applications. Mod. Sup. Abs. Polym. Tech. 1998, 37, 4267–4274. [Google Scholar]
- Zohuriaan, M.J.; Kabiri, K. Superabsorbent Polymer Materials: A Review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Henderson, J.C.; Hensley, D.L. Ammonium and nitrate retention by a hydrophilic gel. Hort. Sci. 1985, 20, 667–668. [Google Scholar]
- Ingram, D.L.; Yeager, T.H. Effect of irrigation frequency and a water-absorbing polymer amendment on Ligustrum growth and moisture retention by a container medium. J. Environ. Hort. 1987, 5, 19–21. [Google Scholar]
- Wang, Y.T.; Boogher, C.A. Effect of a medium-incorporated hydrogel on plant growth and water use of two foliage species. J. Environ. Hort. 1987, 5, 127–130. [Google Scholar]
- Wang, Y.T.; Gregg, L.L. Hydrophilic polymers—Their response to soil amendments and effect on properties of a soil less potting mix. J. Am. Soc. Hort. Sci. 1990, 115, 943–948. [Google Scholar]
- Kabiri, K.; Zohuriaan-Mehr, M.J.; Mirzadeh, H.; Kheirabadi, M.J. Solvent-, ion- and pH-specific swelling of poly (2-acrylamido-2-methyl propane sulfonic acid) super absorbing gels. J. Polym. Res. 2010, 17, 203–212. [Google Scholar] [CrossRef]
- Bulut, Y.; Akcay, G.; Elma, D.; Serhatli, I.E. Synthesis of clay-based superabsorbent composite and its sorption capability. J. Hazard. Mater. 2009, 171, 717–723. [Google Scholar]
- Zhang, J.P.; Wang, A.Q. Study on superabsorbent composites. IX: Synthesis, characterization and swelling behaviors of polyacrylamide/clay composites based on various clays. React. Func. Polym. 2007, 67, 737–745. [Google Scholar] [CrossRef]
- Chu, M.; Zhu, S.Q.; Huang, Z.B.; Li, H.M. Influence of potassium humate on the swelling properties of a poly(acrylic acid-co-acrylamide)/potassium humate superabsorbent composite. J. Appl.Polym. Sci. 2008, 107, 3727–3733. [Google Scholar]
- Liu, J.H.; Wang, Q.; Wang, A.Q. Synthesis and characterization of chitosan-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2007, 70, 166–173. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Li, A.; Wang, A. Preparation, Swelling Behaviors, and Slow-Release Properties of a Poly(acrylic acid-co-acrylamide)/Sodium Humate Superabsorbent Composite. Ind. Eng. Chem. Res. 2006, 45, 48–54. [Google Scholar]
- Zhang J.P.; Li, A.; Wang, A.Q. Study on superabsorbent composite. V. Synthesis, swelling behaviors and application of poly(acrylic acid-co-acrylamide)/sodium humate/attapulgite superabsorbent composite. Polym. Adv. Technol. 2005, 16, 813–820. [Google Scholar]
- Seki, Y.A.; Torgürsül, A.; Yurdakoc, K. Preparation and characterization of poly(acrylic acid)-iron rich smectite superabsorbent composites. Polym. Adv. Technol. 2007, 18, 477–482. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel nanocomposite based on starch-g-poly(sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 2012, 19, 1225–1237. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Neto, A.G.V.C.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, E.A.F.; Muniz, E.C. Nano composites based on poly(acrylamide-co acrylate) and cellulose nanowhiskers. Eur. Polym. J. 2012, 48, 454–463. [Google Scholar]
- Cakmak, I. Identification and Correction of Widespread Zn Deficiency in Turkey: A Success Story; International Fertiliser Society: York, UK, 2004. [Google Scholar]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2rd ed; IZA: Brussels, Belgium and IFA: Paris, France, 2008. [Google Scholar]
- Shaver, T.; Westfall, D.G. Validity of Zinc Fertilizer Efficiency Ratios; Colorado State University: Fort Collins, CO, USA, 2008; Technical Bulletin TB-08-04. [Google Scholar]
- Spagnol, C.; Rodrigues, F.H.A.; Neto, A.G.V.C.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composte made of cellulose nanofibrils and chitosin-g-poly (acrylic acid). Carbohydr. Polym 2011. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, H.; Liu, B.; Wu, Y.; Song, J. Effects of super-absorbent polymers on the physical and chemical properties of soil following different wetting and drying cycles. Soil Use Manag. 2010, 26, 253–260. [Google Scholar] [CrossRef]
- Dorraji, S.S.; Golchin, A.; Ahmadi, S. The effects of hydrophilic polymer and soil salinity on corn growth in sandy and loamy soils. Clean-Soil Air Water 2010, 38, 584–591. [Google Scholar]
- El-Shafei, Y.Z.; Al-Omran, A.M.; Al-Darby, A.M.; Shalaby, A.A. Influence of upper layer treatment of gel-conditioner on water movement in sandy soils under sprinkler infiltration. Arid Soil Res. Rehab. 1992, 6, 217–231. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Shainberg, I.; Goldstein, D.; Warrington, D.N.; Levy, G.J. Water retention and hydraulic conductivity of crosslinked polyacrylamides in sandy soils. Soil Sci. Soc. Am. J. 2007, 71, 406–412. [Google Scholar]
- Taylor, K.C.; Halfacre, R.G. The effect of hydrophilic polymer on media water retention and nutrient availability to Ligustrum lucidum. Hort. Sci. 1986, 21, 1159–1161. [Google Scholar]
- Liu, Z.S.; Rempel, G.L. Preparation of superabsorbent polymers by crosslinking acrylic acid and acrylamide copolymers. J. Appl. Polym. Sci. 1997, 64, 1345–1353. [Google Scholar]
- Moutier, M.; Shainberg, I.; Levy, G.J. Hydraulic gradient and wetting rate effects on the hydraulic conductivity of two calcium vertisols. Soil Sci. Soc. Am. J. 2000, 64, 1211–1219. [Google Scholar]
- Sample Availability: Samples of the compounds, AlZnFe2O4 and poly(Acrylamide-co-acrylic acid)/AlZnFe2O4 can be available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shahid, S.A.; Qidwai, A.A.; Anwar, F.; Ullah, I.; Rashid, U. Improvement in the Water Retention Characteristics of Sandy Loam Soil Using a Newly Synthesized Poly(acrylamide-co-acrylic Acid)/AlZnFe2O4 Superabsorbent Hydrogel Nanocomposite Material. Molecules 2012, 17, 9397-9412. https://doi.org/10.3390/molecules17089397
Shahid SA, Qidwai AA, Anwar F, Ullah I, Rashid U. Improvement in the Water Retention Characteristics of Sandy Loam Soil Using a Newly Synthesized Poly(acrylamide-co-acrylic Acid)/AlZnFe2O4 Superabsorbent Hydrogel Nanocomposite Material. Molecules. 2012; 17(8):9397-9412. https://doi.org/10.3390/molecules17089397
Chicago/Turabian StyleShahid, Shaukat Ali, Ansar Ahmad Qidwai, Farooq Anwar, Inam Ullah, and Umer Rashid. 2012. "Improvement in the Water Retention Characteristics of Sandy Loam Soil Using a Newly Synthesized Poly(acrylamide-co-acrylic Acid)/AlZnFe2O4 Superabsorbent Hydrogel Nanocomposite Material" Molecules 17, no. 8: 9397-9412. https://doi.org/10.3390/molecules17089397
APA StyleShahid, S. A., Qidwai, A. A., Anwar, F., Ullah, I., & Rashid, U. (2012). Improvement in the Water Retention Characteristics of Sandy Loam Soil Using a Newly Synthesized Poly(acrylamide-co-acrylic Acid)/AlZnFe2O4 Superabsorbent Hydrogel Nanocomposite Material. Molecules, 17(8), 9397-9412. https://doi.org/10.3390/molecules17089397






