2.1. Results
Quercetin was dissolved (2 × 10
−5 M) in ethanol and irradiated with increasing doses of UVA.
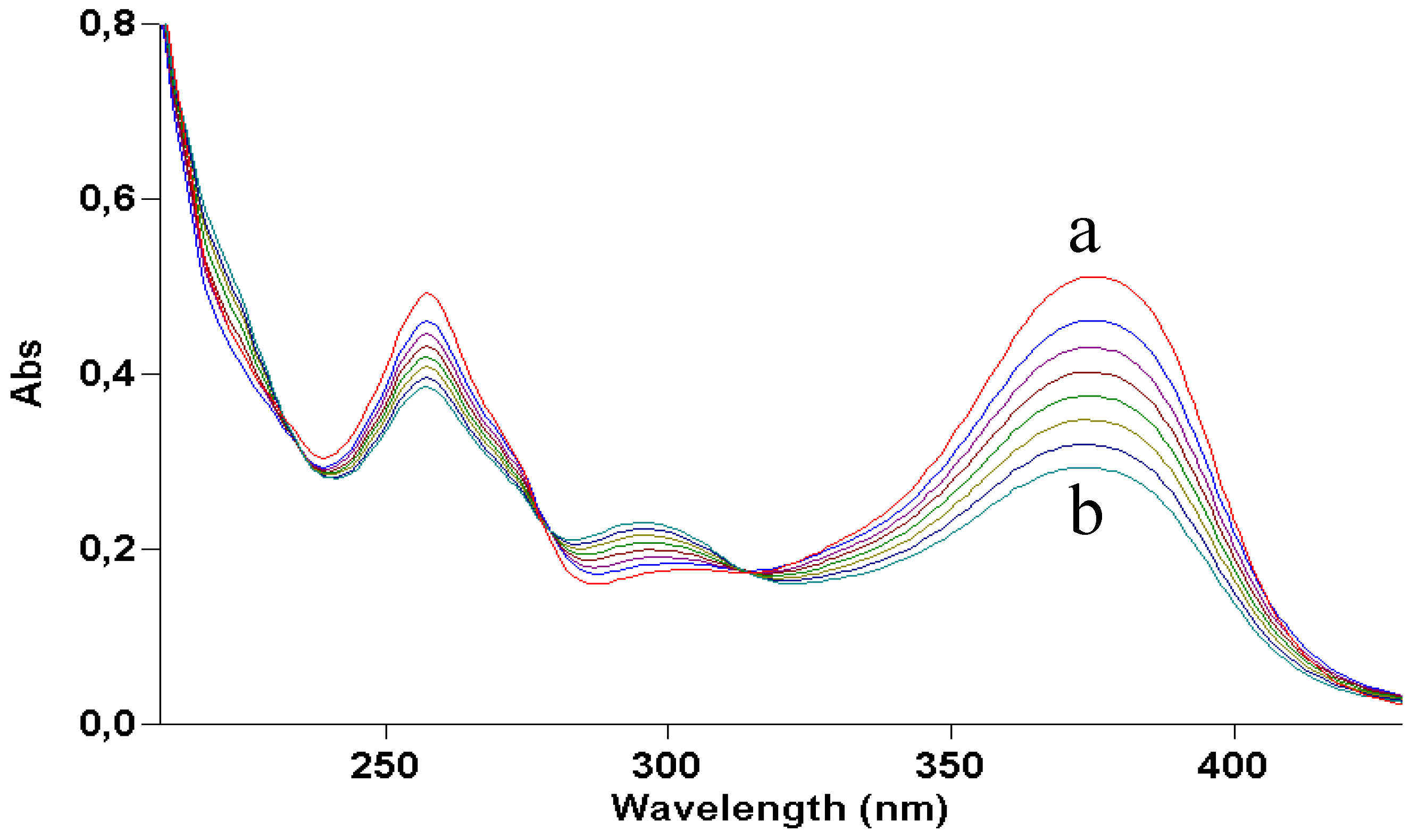
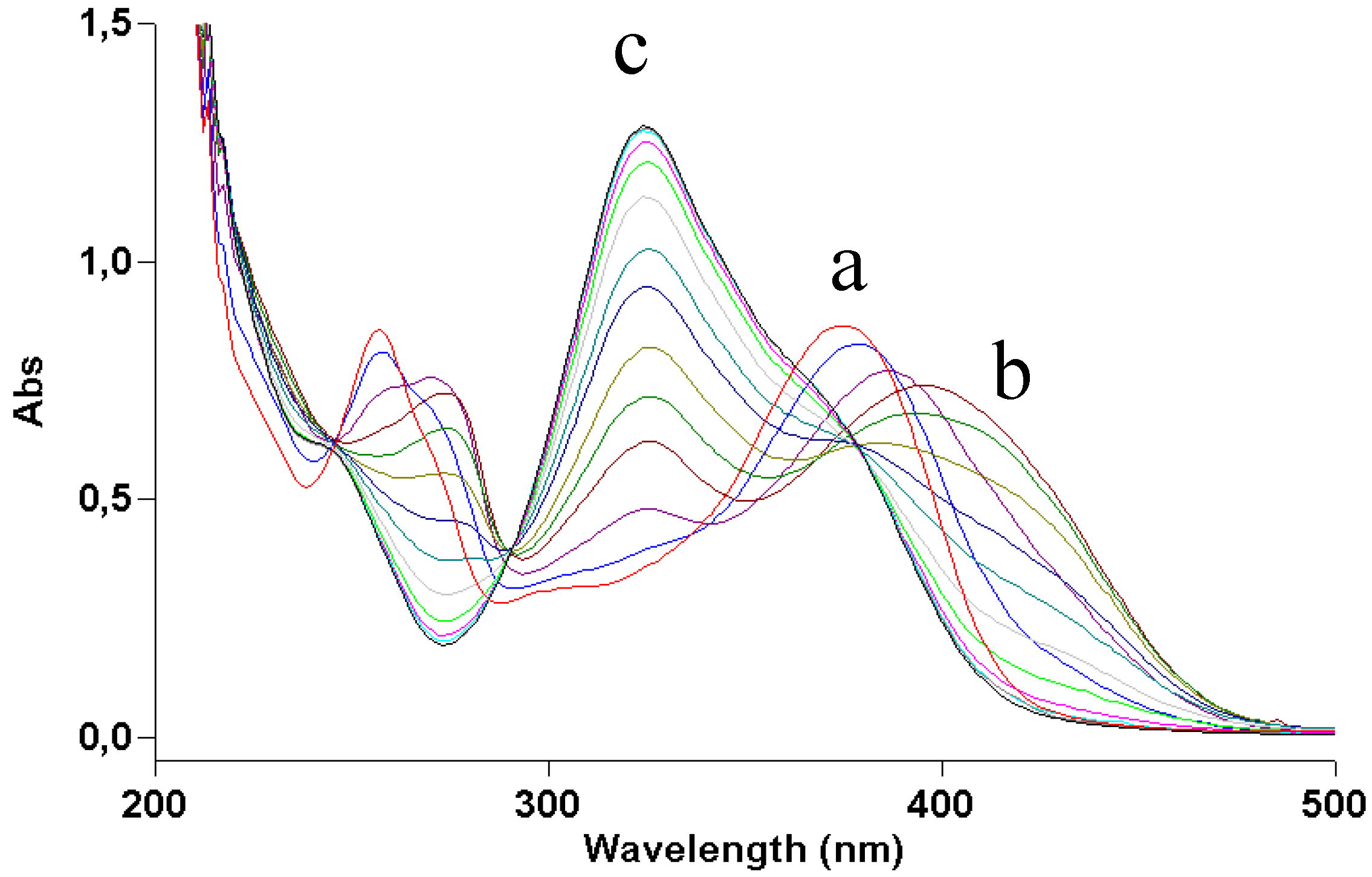
Figure 2 shows how irradiation affects the UV absorption spectrum: the maximum at 375 nm undergoes a gradual blue shift to 295 nm, indicating loss of conjugation of the chromophore. The presence of at least two isosbestic points at 275 and 325 nm suggests that at least one or more photoproducts form upon UV light. During irradiation, the solution was also submitted to negative-ion MS analysis.
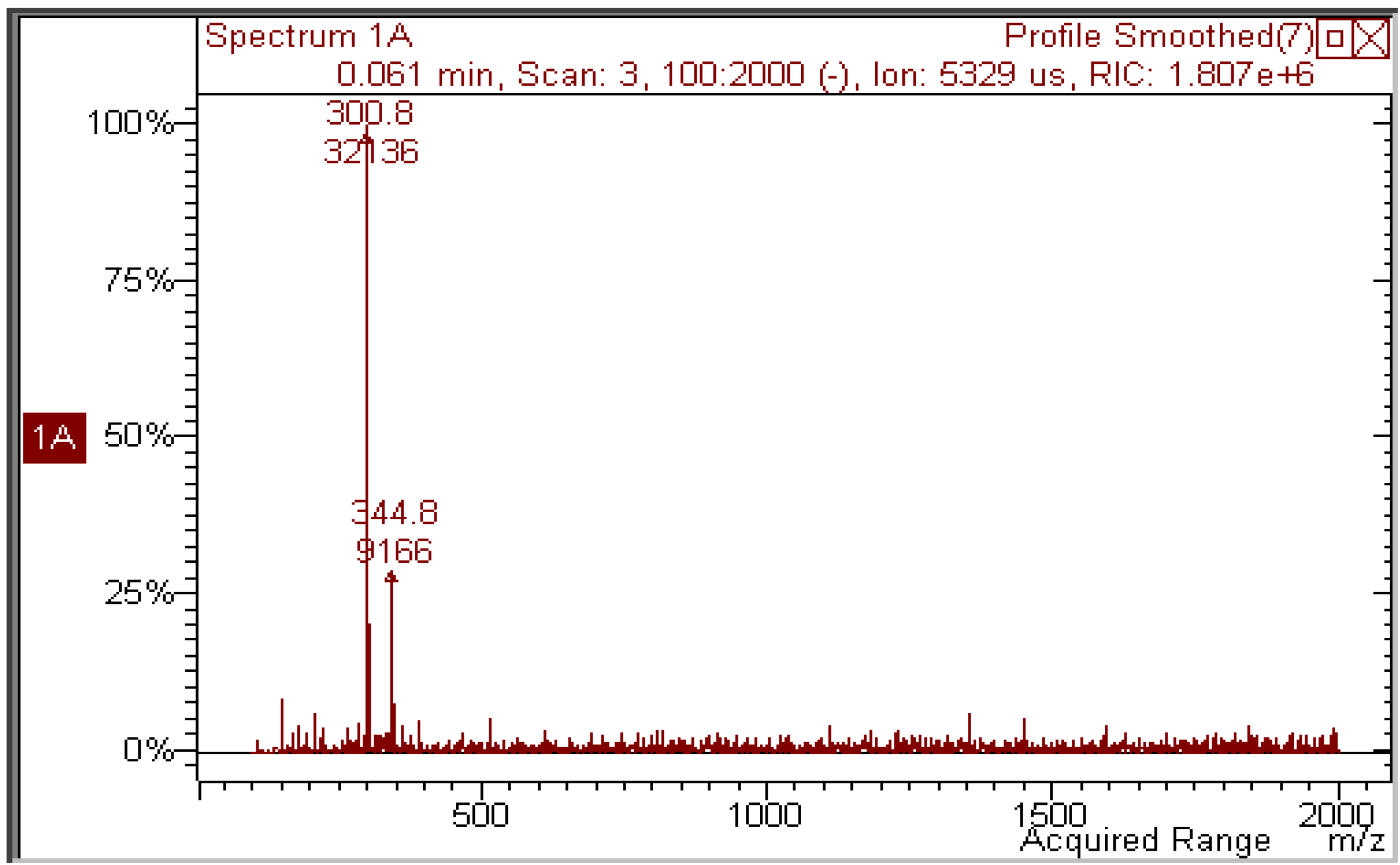
Figure 3 shows that, in the MS spectrum of a sample irradiated with 50 J/cm
2, only two important peaks appear: the first (
m/z 300.8) corresponds to the molecular ion of quercetin [M−H]
− and the second has
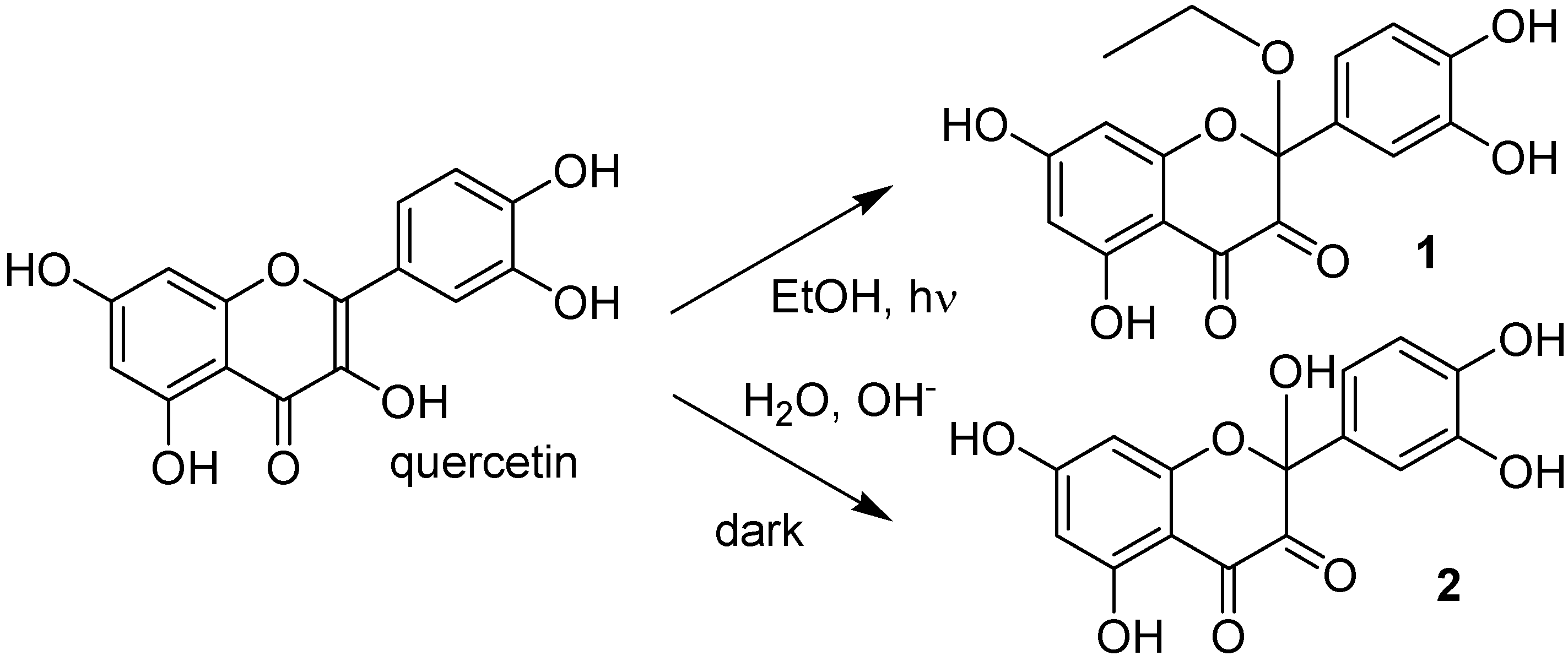
m/z 344.8, corresponding to a molecular weight of 346 Da. The increase in mw of 44 Da corresponds to the addition of an ethanol molecule to the 2,3 double bond of quercetin (thus explaining the blue shift in the UV absorption spectrum), accompanied by oxidative loss of two hydrogen atoms (compound
1 in
Scheme 1). This modification was substantiated by further experiments: (1) removal of oxygen from the solution through bubbling with argon for 10 min before and during irradiation completely abolished photolysis, as did the addition of a small amount of a concentrated aqueous solution of ascorbic acid; (2) when methanol was used, the mw of the photoproduct was 332 (quercetin + MeOH − 2H), but when deuterated methanol was added, the mw was 335, showing that the −OCD
3 moiety was present in the product; the fourth deuterium atom was lost in the oxidative step. In addition, the proposed structure corresponds to that already obtained by Zhou
et al. [
13] by electrochemical oxidation of quercetin in ethanol.
Figure 2.
UV absorption spectra of a 2 × 10−5 M ethanol solution of quercetin irradiated with increasing doses of UVA. Curve a, dark; curve b, 70 J/cm2.
Figure 2.
UV absorption spectra of a 2 × 10−5 M ethanol solution of quercetin irradiated with increasing doses of UVA. Curve a, dark; curve b, 70 J/cm2.
Experiments were repeated with UVB light, but no significant differences were found in either products or rate of photodegradation. In a non-nucleophilic solvent like acetonitrile, quercetin was stable. Attempts were made to remove the solvent in order to perform NMR analysis on the photoproduct. Unfortunately, even mild conditions like lyophilization were unsuccessful, because of photoproduct instability.
Figure 3.
Negative-ion mass spectrum of a 2 × 10−5 M solution of quercetin in ethanol irradiated with 50 J/cm2 UVA.
Figure 3.
Negative-ion mass spectrum of a 2 × 10−5 M solution of quercetin in ethanol irradiated with 50 J/cm2 UVA.
Scheme 1.
Proposed structure of quercetin products obtained by either UVA irradiation in ethanol (1) or in aqueous ammonia in the dark (2).
Scheme 1.
Proposed structure of quercetin products obtained by either UVA irradiation in ethanol (1) or in aqueous ammonia in the dark (2).
Quercetin is practically insoluble in water. To study its photostability in an aqueous environment, a water suspension of the compound was made slightly alkaline (pH ≈ 9) by adding ammonia. However, as already reported [
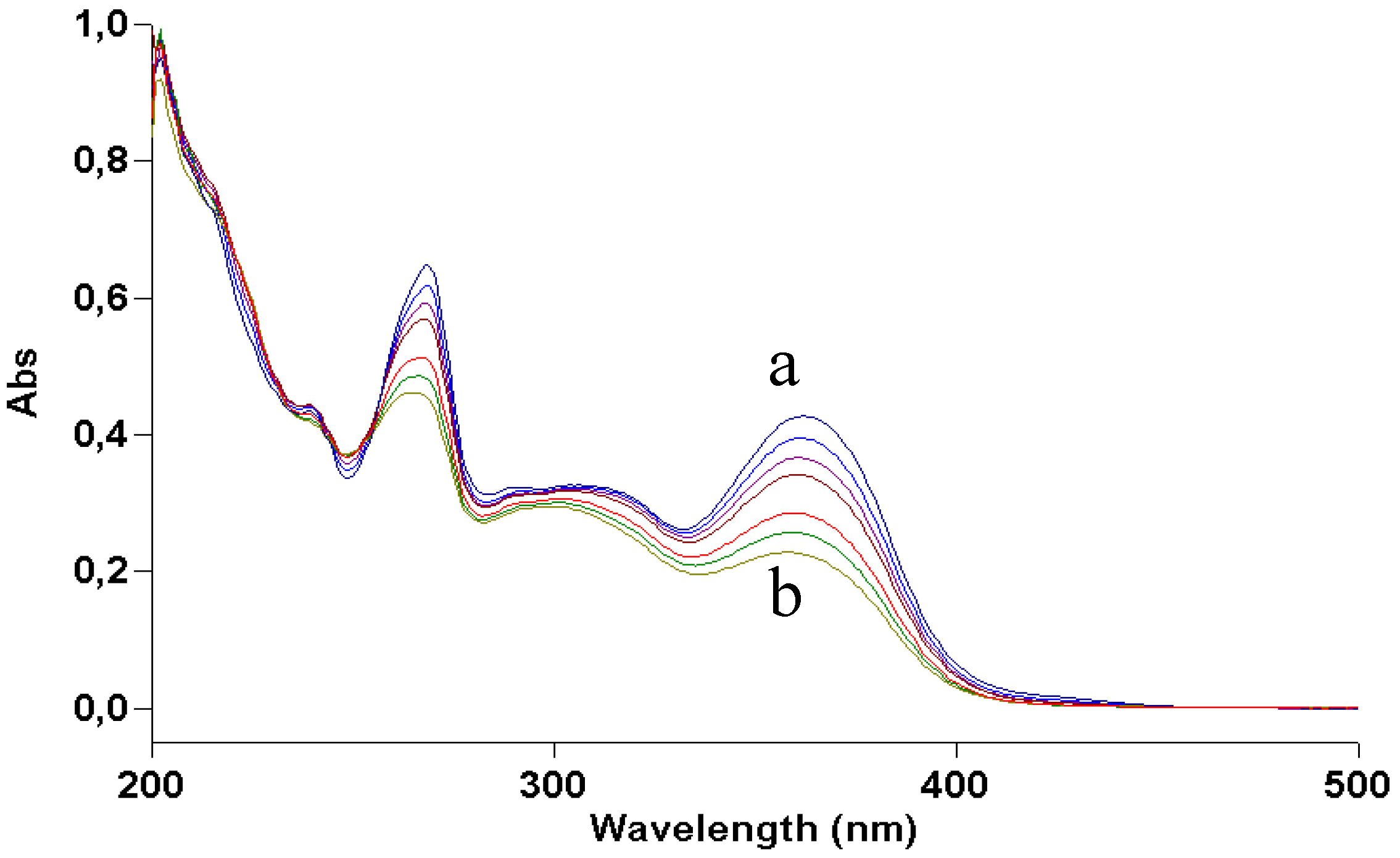
10], this treatment leads to degradation of the molecule in the dark. Recording fast UV absorption spectra at 5-s intervals gave the results shown in
Figure 4. During the first few seconds the spectrum showed a red shift due to ionization of the molecule, quickly followed by a blue shift. The final profile closely resembled that of quercetin irradiated in ethanol (
Figure 2), suggesting a similar structure. In this case too, careful removal of oxygen or addition of ascorbic acid stopped the reaction at the ionization step (UV absorption maximum at 395 nm).
As observed by Zenkevich
et al. [
10] and with the photoproduct formed in ethanol (see above), the product was thermally unstable, making its isolation impossible. However, MS measurements on the crude solution revealed that a single photoproduct was formed, with a mw of 318 Da (quercetin + H
2O − 2H). When deuterium oxide was used as the solvent, the mw was 319, allowing the product to be assigned to structure
2 (
Scheme 1).
Figure 4.
Time-course of UV absorption spectrum of a solution of quercetin in aqueous ammonia. Curve a was recorded immediately after addition of ammonia, curve b after 15 s, curve c after 60 s.
Figure 4.
Time-course of UV absorption spectrum of a solution of quercetin in aqueous ammonia. Curve a was recorded immediately after addition of ammonia, curve b after 15 s, curve c after 60 s.
The latter experiment—that is, alkalinization of a suspension of quercetin in deuterium oxide—was also possible with higher amounts of quercetin, suitable for
13C-NMR experiments:
Table 1 compares the spectroscopic data of the product with those of parent quercetin. Only two carbon atoms differ significantly in their resonance between quercetin and the product,
i.e., C-2 and C-3, matching the proposed structure. Instead, the
1H-NMR spectrum showed that the chemical shift of the non-OH protons, H-6, 7, 2′, 5′, and 6′, was not significantly changed.
Table 1.
13C-NMR spectroscopic data (75 MHz) for quercetin and product 2.
Table 1.
13C-NMR spectroscopic data (75 MHz) for quercetin and product 2.
| Position | Quercetin (in CD3OD) | Product 2 (in D2O + NH3) |
|---|
| 2, qC | 147.5 | 113.7 |
| 3, qC | 136.5 | 180.2 |
| 4, qC | 176.5 | 174.4 |
| 5, qC | 161.0 | 157.9 |
| 6, CH | 99.5 | 101.7 |
| 7, qC | 166.0 | 159.8 |
| 8, CH | 94.5 | 96.3 |
| 9, qC | 156.7 | 154.5 |
| 10, qC | 104.0 | 102.0 |
| 1′, qC | 123.0 | 121.6 |
| 2′, CH | 116.0 | 116.9 |
| 3′, qC | 145.7 | 141.9 |
| 4′, qC | 148.1 | 149.3 |
| 5′, CH | 116.5 | 117.2 |
| 6′, CH | 121.0 | 120.3 |
Both photolysis of quercetin in alcohols and degradation in alkalis thus proceed through two steps: (i) addition of solvent; and (ii) oxidation (not necessarily in this order, see Discussion).
Further support to the similarity of compounds 1 and 2 came from MS/MS experiments: in both, the first fragmentation was the loss of the group in position 3 (OEt or OH, respectively) followed by the same fragmentation pattern.
For oxidation, the presence of a hydroxy group in position 3 seems to be mandatory. Four flavonoid analogs of quercetin were then studied (
Figure 1). In apigenin, the hydroxy group was substituted by a hydrogen atom; in rutin it was glycosylated. Both compounds, dissolved in methanol, proved to be stable under UV irradiation. When dissolved in aqueous ammonia, both underwent deprotonation—with consequent redshift of the UV absorption spectrum—but no further degradation occurred.
Kaempferol and galangin (
Figure 1), which lack one or both hydroxy groups of ring B while maintaining the hydroxy group in 3, were expected to behave like quercetin. This was only partly true for the former (
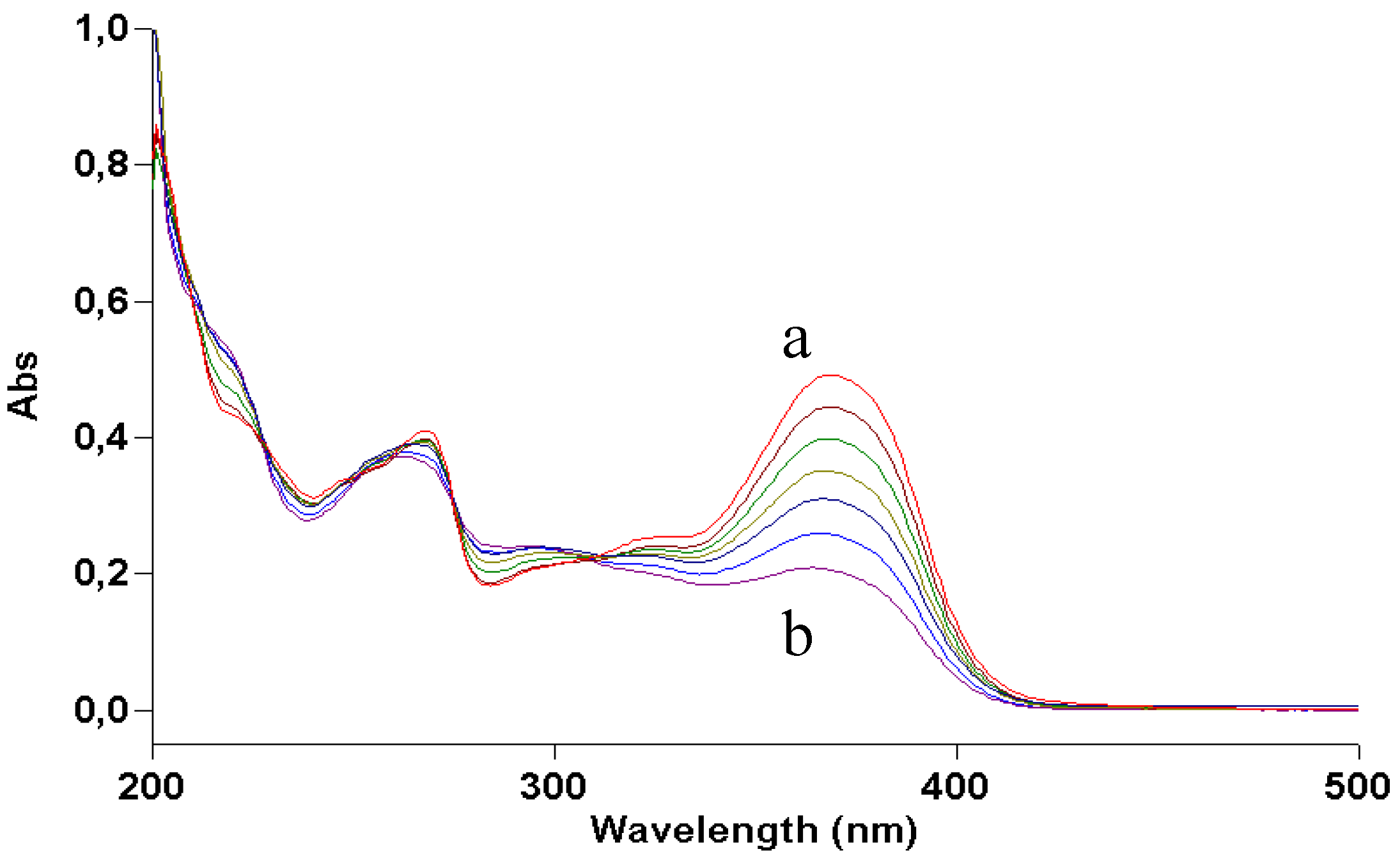
Figure 5): comparison of its photolysis in methanol with that of quercetin (
Figure 2) shows a similar decrease in absorption at 380 nm, whereas both decrease at 280 and increase at 300 are less definite, like the isosbestic points. The mass spectrum (
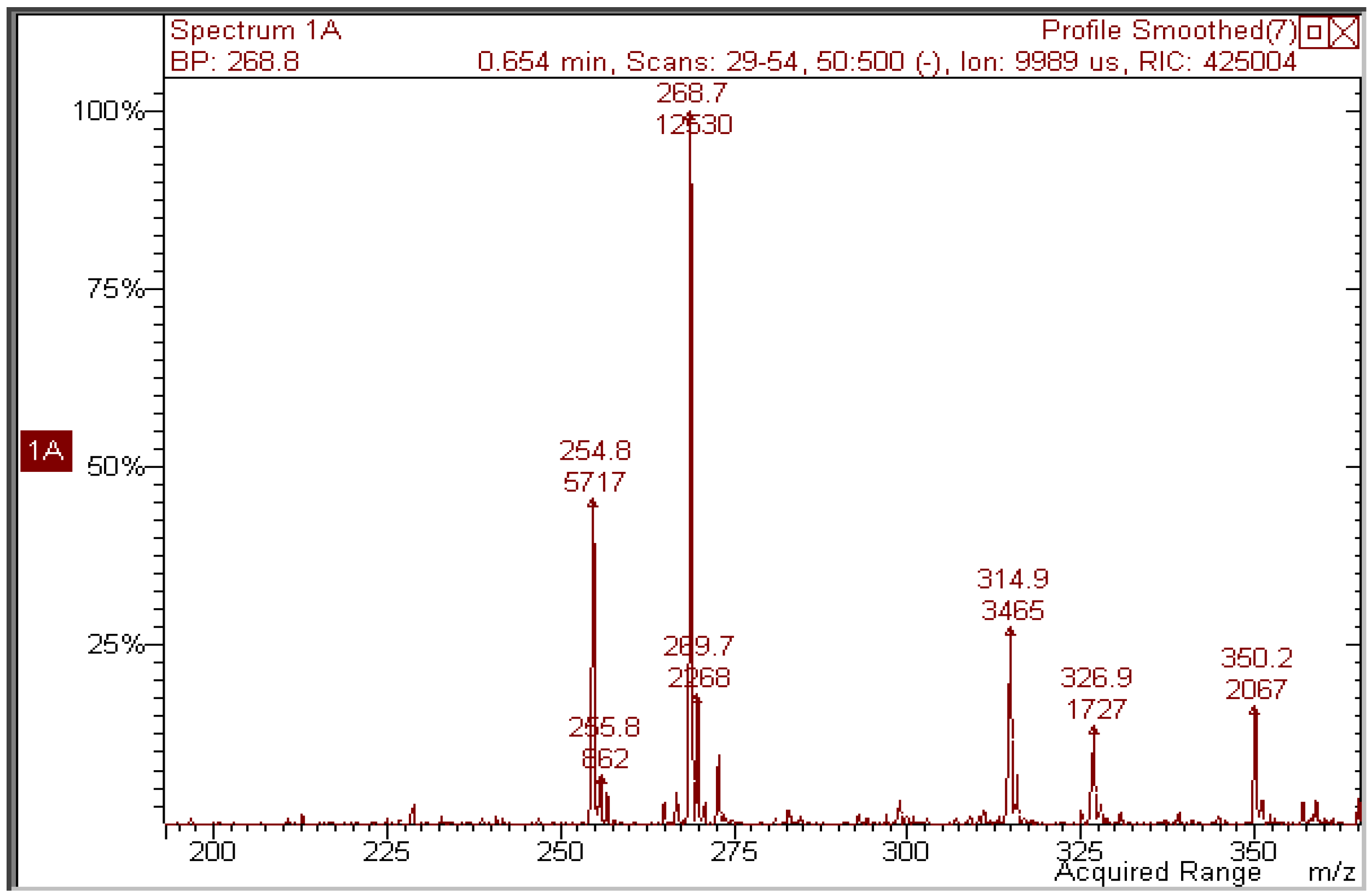
Figure 6) does show that the peak of the expected photoproduct at
m/z 314.9 is a minor signal with respect to other unidentified ones.
Figure 5.
UV absorption spectra of a 2 × 10−5 M methanol solution of kaempferol irradiated with increasing doses of UVA. Curve a, dark; curve b, 70 J/cm2.
Figure 5.
UV absorption spectra of a 2 × 10−5 M methanol solution of kaempferol irradiated with increasing doses of UVA. Curve a, dark; curve b, 70 J/cm2.
Figure 6.
Negative-ion mass spectrum of a 2 × 10−5 M methanol solution of kaempferol irradiated with 70 J/cm2 UVA.
Figure 6.
Negative-ion mass spectrum of a 2 × 10−5 M methanol solution of kaempferol irradiated with 70 J/cm2 UVA.
Results obtained by dissolving kaempferol and galangin in aqueous ammonia were consistent with the irradiation experiments. In the former, the product deriving from water addition and oxidation was present, although mixed with several byproducts; the latter was only deprotonated without further degradation (
Figure 7 and
Figure 8).
Figure 7.
UV absorption spectra of a 2 × 10−5 M methanol solution of galangin irradiated with increasing doses of UVA. Curve a, dark; curve b, 70 J/cm2.
Figure 7.
UV absorption spectra of a 2 × 10−5 M methanol solution of galangin irradiated with increasing doses of UVA. Curve a, dark; curve b, 70 J/cm2.
Figure 8.
Negative-ion mass spectrum of a 2 × 10−5 M methanol solution of galangin irradiated with 70 J/cm2 UVA.
Figure 8.
Negative-ion mass spectrum of a 2 × 10−5 M methanol solution of galangin irradiated with 70 J/cm2 UVA.
2.2. Discussion
The photostability of quercetin in nucleophilic solvents (MeOH and EtOH) was studied. One photoproduct (
1 in
Scheme 1) was characterized by mass spectrometry as coming from addition of the alcohol to the 2,3 double bond of the flavone and oxidation by air, but proved unstable to isolation procedures. Addition may precede oxidation or
vice versa. On the one hand, in the former hypothesis the structure of the possible quercetin-ethanol adduct closely resembles that of ascorbic acid: if addition occurs first, oxidation should then be easy. On the other hand, studying the cooperation between quercetin and ascorbate on the suppression of photosensitized hemolysis, Sorata
et al. [
14] found that ascorbate reduced the oxidized form of quercetin, rather than acting as a singlet oxygen and radical quencher. There are other examples of photoaddition of nucleophiles to the vinyl bond of β-unsaturated ketones. To mention only one, the 5,6 double bond of the antitumor agent 5-fluorouracil adds water on irradiation [
15]. However, rutin, which has a non-oxidizable electron-drawing group in position 3, like 5-fluorouracil, is photostable, thus ruling out the possibility that addition is the first step of the process.
As regards the behaviour of the flavones in alkaline medium, Zenkevich
et al. [
10] were not able to characterize the primary photoproduct because of its instability. On the basis of the final degradation products (floroglucinol, 3,4-dihydroxybenzoic acid, and 2,4,6-trihydroxybenzoic acid) they proposed an intermediate of depsidic nature, in which ring C is open. In the present paper, by means of MS and
13C-NMR, the structure of the primary product (compound
2 in
Scheme 1) was shown to be similar to that obtained with light: it derives from oxidation and the addition of water. The depside is probably formed from
2 in a later step, during the removal of the solvent, as the final products mentioned above were also found in this study.
Kaempferol and galangin have lower antioxidant properties than quercetin [
16]. In fact, both under irradiation in methanol and in the dark in alkaline medium, their behaviour is different from that of quercetin: slightly different when one hydroxy group still remains on ring C (kaempferol), and drastically, when both hydroxy groups are removed (galangin).
As
Figure 2 shows, the light dose necessary to destroy quercetin is very high: about 70 J/cm
2 for 50% degradation. The quantum yield of photodegradation was roughly estimated to be of the order of 10
−4. The percentage of modified molecules therefore becomes negligible in more concentrated solution. This fact may explain the stability of quercetin observed by Choquenet [
5], as it was measured at a concentration as high as 10% (w/w), necessary to achieve a suitable sun protection factor. Thus, the photodegradation of quercetin should not be a problem in such conditions.