Synthesis and Sensory Evaluation of ent-Kaurane Diterpene Glycosides
Abstract
:1. Introduction
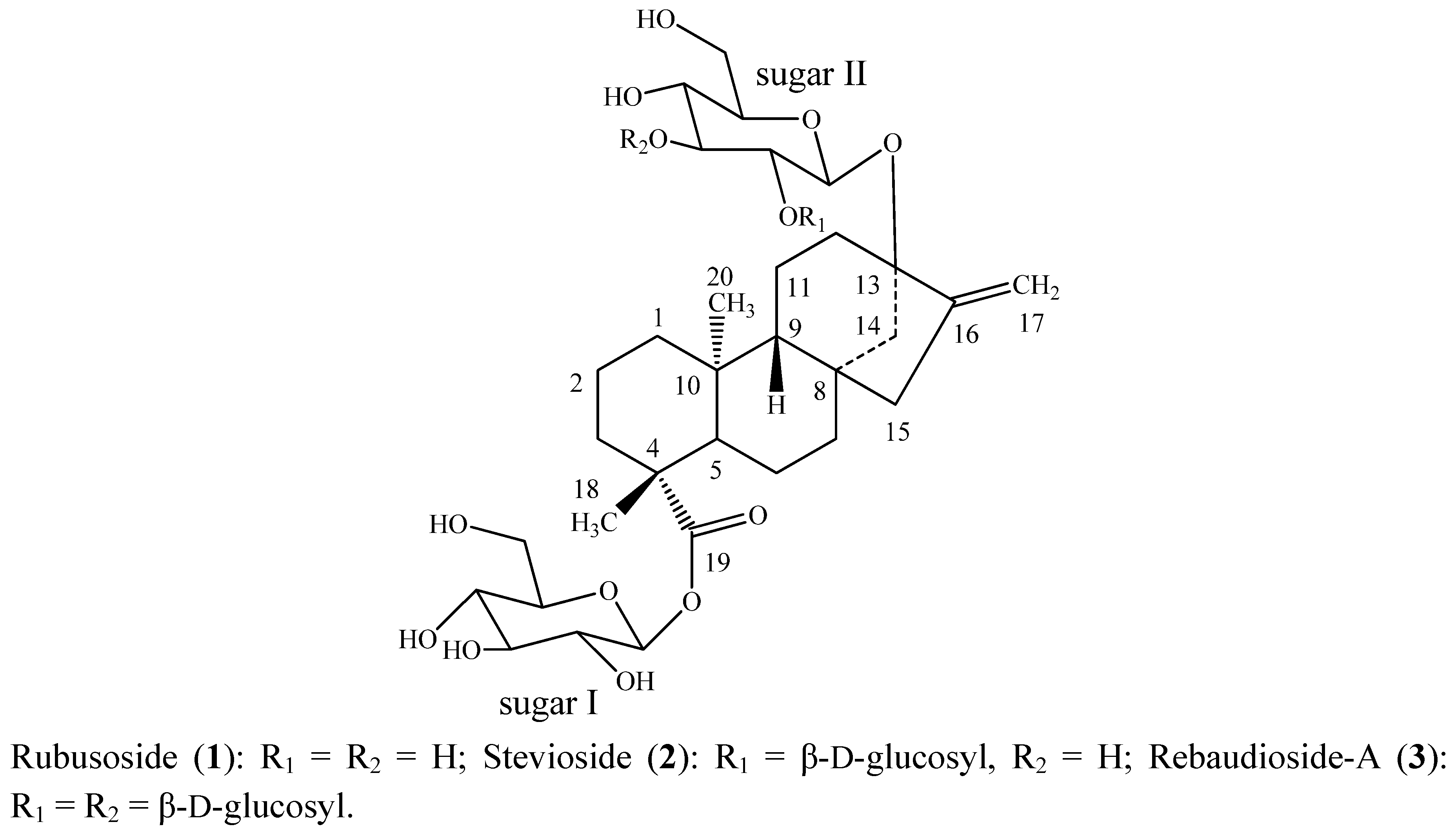
2. Results and Discussion
2.1. Chemistry and Sensory Studies
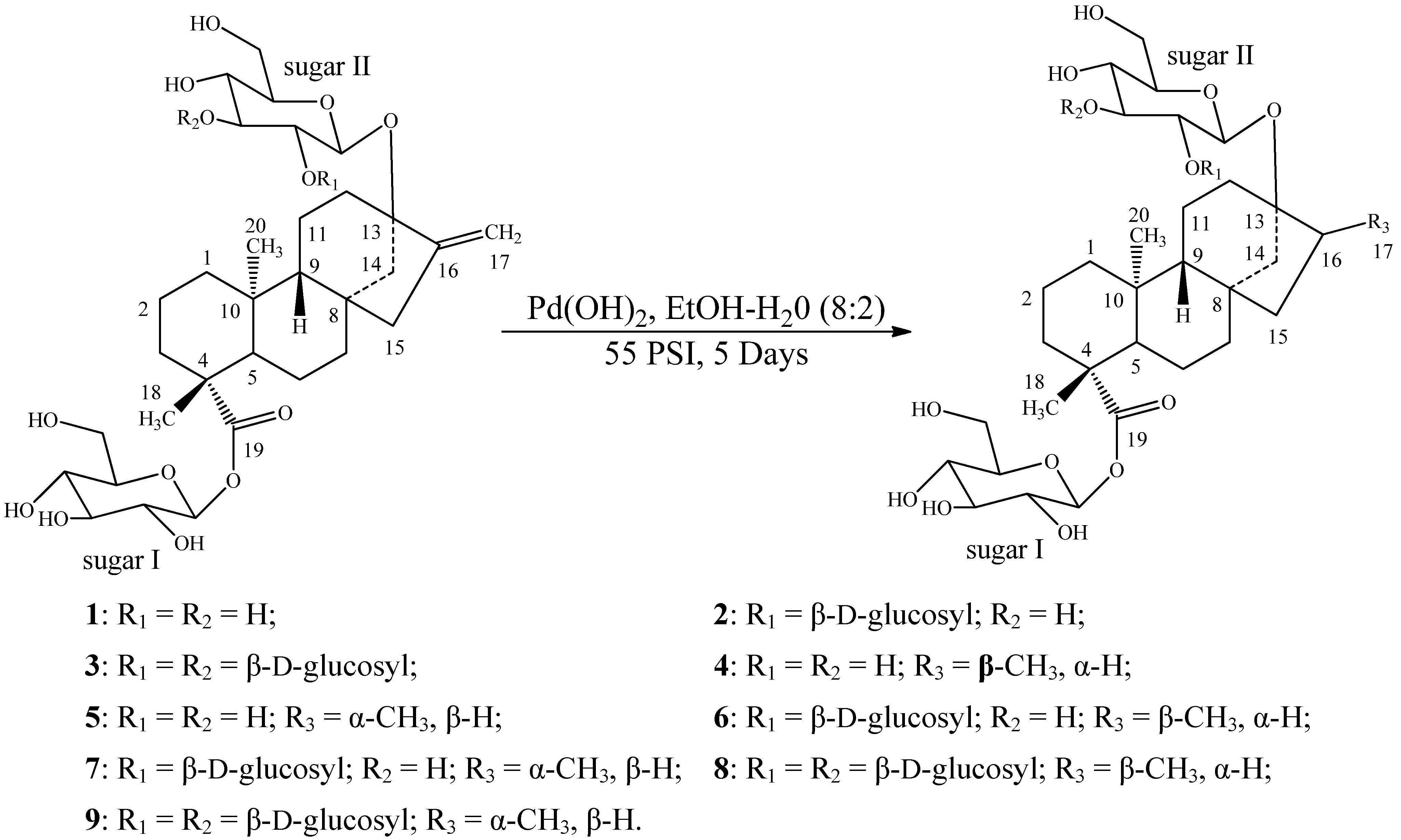
2.2. Spectroscopy
| Steviol Glycoside Type | Sensory Evaluation of Original Compound | Sensory Evaluation of Reduced Compound |
|---|---|---|
| Rubusoside | Slow onset of sweetness, about 2–3% sucrose equivalence | No sweetness |
| Stevioside | Slow onset of sweetness, sweet lingering aftertaste, about 5–6% sucrose equivalence | Slow onset of sweetness, less sweetness linger to original, about 2–3% sucrose equivalence |
| Rebaudioside A | Slow onset of sweetness, sweet lingering aftertaste, about 6–7% sucrose equivalence | Slow onset of sweetness, less sweetness linger to original, about 3–4% sucrose equivalence |
| Position | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|
| 17 | 1.10 (d, 6.4, 1H) | 1.16 (d, 6.5, 1H) | 1.21 (d, 6.7, 1H) | 1.28 (d, 6.4, 1H) | 1.11 (d, 6.5, 1H) | 1.17 (d, 6.5, 1H) |
| 18 | 1.25 (s, 3H) | 1.25 (s, 3H) | 1.25 (s, 3H) | 1.25 (s, 3H) | 1.25 (s, 3H) | 1.25 (s, 3H) |
| 20 | 1.32 (s, 3H) | 1.32 (s, 3H) | 1.29 (s, 3H) | 1.29 (s, 3H) | 1.31 (s, 3H) | 1.31 (s, 3H) |
| 1′ | 6.16 (d, 6.8, 1H) | 6.14 (d, 6.6, 1H) | 6.15 (d, 6.8, 1H) | 6.15 (d, 6.5, 1H) | 6.15 (d, 6.5, 1H) | 6.17 (d, 6.5, 1H) |
| 1′′ | 5.03 (d, 6.7, 1H) | 5.02 (d, 6.6, 1H) | 5.09 (d, 6.7, 1H) | 5.04 (d, 6.9, 1H) | 5.00 (d, 6.7, 1H) | 5.03 (d, 6.9, 1H) |
| 1′′′ | 5.25 (d, 6.5, 1H) | 5.27 (d, 6.8, 1H) | 5.35 (d, 6.6, 1H) | 5.32 (d, 6.5, 1H) | ||
| 1′′′′ | 5.52 (d, 6.4, 1H) | 5.44 (d, 6.8, 1H) |
| Position | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|
| 1 | 41.3 | 41.3 | 41.2 | 41.2 | 41.2 | 41.2 |
| 2 | 20.4 | 20.3 | 20.1 | 20.1 | 20.1 | 20.1 |
| 3 | 38.9 | 38.8 | 38.9 | 38.8 | 38.9 | 38.8 |
| 4 | 45.1 | 43.0 | 44.8 | 43.1 | 44.9 | 43.0 |
| 5 | 57.9 | 57.9 | 57.8 | 57.8 | 57.8 | 57.8 |
| 6 | 22.8 | 23.2 | 22.7 | 23.0 | 22.8 | 23.0 |
| 7 | 41.5 | 40.3 | 41.7 | 40.1 | 41.7 | 40.2 |
| 8 | 44.1 | 43.1 | 44.3 | 43.0 | 44.4 | 43.1 |
| 9 | 55.9 | 54.9 | 55.6 | 54.6 | 55.6 | 54.7 |
| 10 | 40.2 | 40.3 | 40.1 | 40.2 | 40.1 | 40.3 |
| 11 | 20.5 | 20.8 | 20.3 | 20.6 | 20.4 | 20.6 |
| 12 | 36.6 | 44.1 | 35.3 | 44.2 | 35.4 | 44.3 |
| 13 | 86.0 | 85.8 | 86.2 | 86.2 | 88.2 | 88.1 |
| 14 | 47.5 | 50.8 | 47.3 | 50.6 | 47.2 | 50.6 |
| 15 | 47.5 | 44.6 | 47.2 | 44.9 | 47.3 | 44.8 |
| 16 | 41.3 | 38.8 | 41.2 | 39.0 | 41.2 | 38.9 |
| 17 | 14.2 | 19.9 | 14.2 | 19.7 | 14.4 | 19.8 |
| 18 | 28.6 | 28.7 | 28.6 | 28.6 | 28.6 | 28.6 |
| 19 | 177.5 | 177.6 | 177.4 | 177.5 | 177.5 | 177.6 |
| 20 | 15.9 | 16.1 | 15.8 | 16.0 | 15.7 | 15.9 |
| 1′ | 96.3 | 96.3 | 96.2 | 96.2 | 96.2 | 96.2 |
| 2′ | 75.9 | 75.9 | 74.3 | 74.4 | 75.7 | 75.6 |
| 3′ | 79.6 | 79.7 | 79.6 | 79.7 | 79.6 | 79.8 |
| 4′ | 71.6 | 71.5 | 71.4 | 71.4 | 71.5 | 71.4 |
| 5′ | 78.3 | 78.5 | 78.2 | 78.4 | 78.5 | 78.6 |
| 6′ | 63.7 | 63.8 | 63.1 | 63.4 | 63.1 | 63.3 |
| 1′′ | 100.4 | 99.8 | 98.3 | 98.4 | 99.0 | 98.8 |
| 2′′ | 74.5 | 74.5 | 84.3 | 84.7 | 78.4 | 78.5 |
| 3′′ | 79.3 | 79.3 | 78.1 | 78.1 | 86.5 | 85.6 |
| 4′′ | 73.0 | 73.2 | 72.9 | 72.9 | 71.9 | 72.0 |
| 5′′ | 79.7 | 79.8 | 79.5 | 79.5 | 77.2 | 77.0 |
| 6′′ | 62.5 | 62.5 | 62.7 | 62.7 | 62.7 | 62.8 |
| 1′′′ | 106.8 | 107.1 | 105.1 | 105.5 | ||
| 2′′′ | 74.3 | 74.3 | 74.6 | 74.6 | ||
| 3′′′ | 77.5 | 77.7 | 77.6 | 77.8 | ||
| 4′′′ | 71.7 | 71.9 | 71.9 | 72.0 | ||
| 5′′′ | 79.2 | 79.2 | 78.9 | 79.0 | ||
| 6′′′ | 62.4 | 62.4 | 62.5 | 62.5 | ||
| 1′′′′ | 105.3 | 105.9 | ||||
| 2′′′′ | 74.3 | 74.4 | ||||
| 3′′′′ | 79.8 | 79.9 | ||||
| 4′′′′ | 72.2 | 72.1 | ||||
| 5′′′′ | 79.6 | 79.7 | ||||
| 6′′′′ | 63.2 | 63.1 |
3. Experimental
3.1. General
3.2. Isolation of Reduced Steviol Glycosides 4–9
3.2.1. General Procedure for the Catalytic Hydrogenation of Steviol Glycosides 1–3
3.2.2. General Procedure for the Enzymatic Hydrolysis of Reduced Steviol Glycoside Mixtures
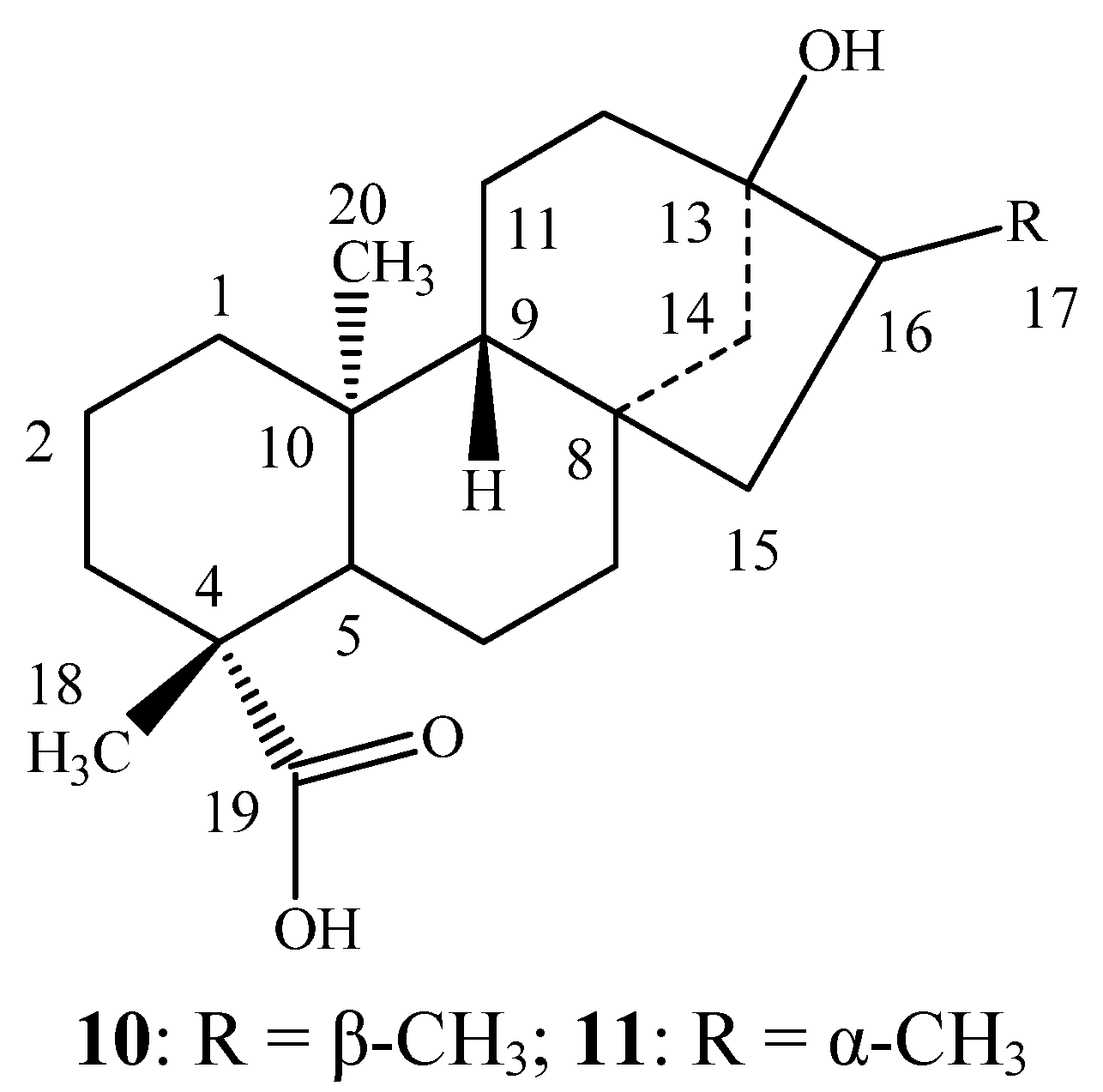
3.3. Sensory Evaluation of the Reduced Steviol Glycoside Mixtures
3.4. Multi-Sip and Swallow Taste Method
4. Conclusions
Acknowledgements
References
- Brandle, J.E.; Starrratt, A.N.; Gijen, M. Stevia rebaudiana: Its agricultural, biological and chemical properties. Can. J. Plant Sci. 1998, 78, 527–536. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Diterpene glycosides from Stevia rebaudiana. J. Med. Plants Res. 2011, 5, 4838–4842. [Google Scholar]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Diterpene glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552–3562. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. A new diterpenoid glycoside from Stevia rebaudiana. Molecules 2011, 16, 2937–2943. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Structures of the novel diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 1057–1060. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two minor diterpene glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 175–178. [Google Scholar]
- Chaturvedula, V.S.P.; Prakash, I. Additional minor diterpene glycosides from Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 1059–1062. [Google Scholar]
- Chaturvedula, V.S.P.; Clos, J.F.; Rhea, J.; Milanowski, D.; Mocek, U.; DuBois, G.E.; Prakash, I. Minor diterpene glycosides from the leaves of Stevia rebaudiana. Phytochem. Lett. 2011, 4, 209–212. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Structures of the novel α-glucosyl linked diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 2034–2038. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Klucik, J.; Mani, U.; Prakash, I. Synthesis of ent-kaurane diterpene glycosides. Molecules 2011, 16, 8402–8409. [Google Scholar]
- Chaturvedula, V.S.P.; Clos, J.F.; Prakash, I. Stability study of steviol glycosides in mock beverages using fluorescent light exposure under ICH guidelines. Int. J. Pharm. Pharm. Sci. 2011, 3, 316–323. [Google Scholar]
- Chaturvedula, V.S.P.; Clos, J.F.; Prakash, I. Stability of steviol glycosides in mock beverages under acidic conditions. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. 5), 421–425. [Google Scholar]
- Prakash, I.; Clos, J.F.; Chaturvedula, V.S.P. Stability of rebaudioside A under acidic conditions and its degradation products. Food Res. Int. 2012, 48, 65–75. [Google Scholar] [CrossRef]
- Mani, U.; DuBois, G.; Prakash, I. Synthetic study on the relationship between structure and sweet taste properties of steviol glycosides. Molecules 2012, 17, 4186–4196. [Google Scholar] [CrossRef]
- Kasai, R.; Kaneda, N.; Tanaka, O.; Yamasaki, K.; Sakamoto, I.; Morimoto, K.; Okada, S.; Kitahata, S.; Furukawa, H. Sweet diterpene glycosides of leaves of Stevia rebaudiana Bertoni: Synthesis and structure-sweetness relation of rebaudiosides A, D, and E and their related glycosides. Nippon Kagaku Kaishi 1981, 5, 726–735. [Google Scholar]
- Nanayakkara, N.P.D.; Klocke, J.A.; Compadre, C.M.; Hussain, R.A.; Pezzuto, J.M.; Kinghorn, A.D. Characteriztaion and feeding deterrent effects on the aphid, Schizaphis graminum, of some derivatives of the sweet compounds, stevioside and rebaudioside A. J. Nat. Prod. 1987, 50, 434–441. [Google Scholar] [CrossRef]
- Pezzuto, J.M.; Compadre, C.M.; Swanson, S.M.; Nanayakkara, N.P.D.; Kinghorn, A.D. Metabolically activated steviol, the aglycone of stevioside, is mutagenic. Proc. Natl. Acad. Sci. USA 1985, 82, 2478–2482. [Google Scholar]
- Kamiya, S.; Konishi, F.; Esaki, S. Synthesis and taste of some analogs of stevioside. Agric. Biol. Chem. 1979, 43, 1863–1867. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the three synthesized steviol glycosides 4–9 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prakash, I.; Campbell, M.; San Miguel, R.I.; Chaturvedula, V.S.P. Synthesis and Sensory Evaluation of ent-Kaurane Diterpene Glycosides. Molecules 2012, 17, 8908-8916. https://doi.org/10.3390/molecules17088908
Prakash I, Campbell M, San Miguel RI, Chaturvedula VSP. Synthesis and Sensory Evaluation of ent-Kaurane Diterpene Glycosides. Molecules. 2012; 17(8):8908-8916. https://doi.org/10.3390/molecules17088908
Chicago/Turabian StylePrakash, Indra, Mary Campbell, Rafael Ignacio San Miguel, and Venkata Sai Prakash Chaturvedula. 2012. "Synthesis and Sensory Evaluation of ent-Kaurane Diterpene Glycosides" Molecules 17, no. 8: 8908-8916. https://doi.org/10.3390/molecules17088908




