Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pollen Analyses
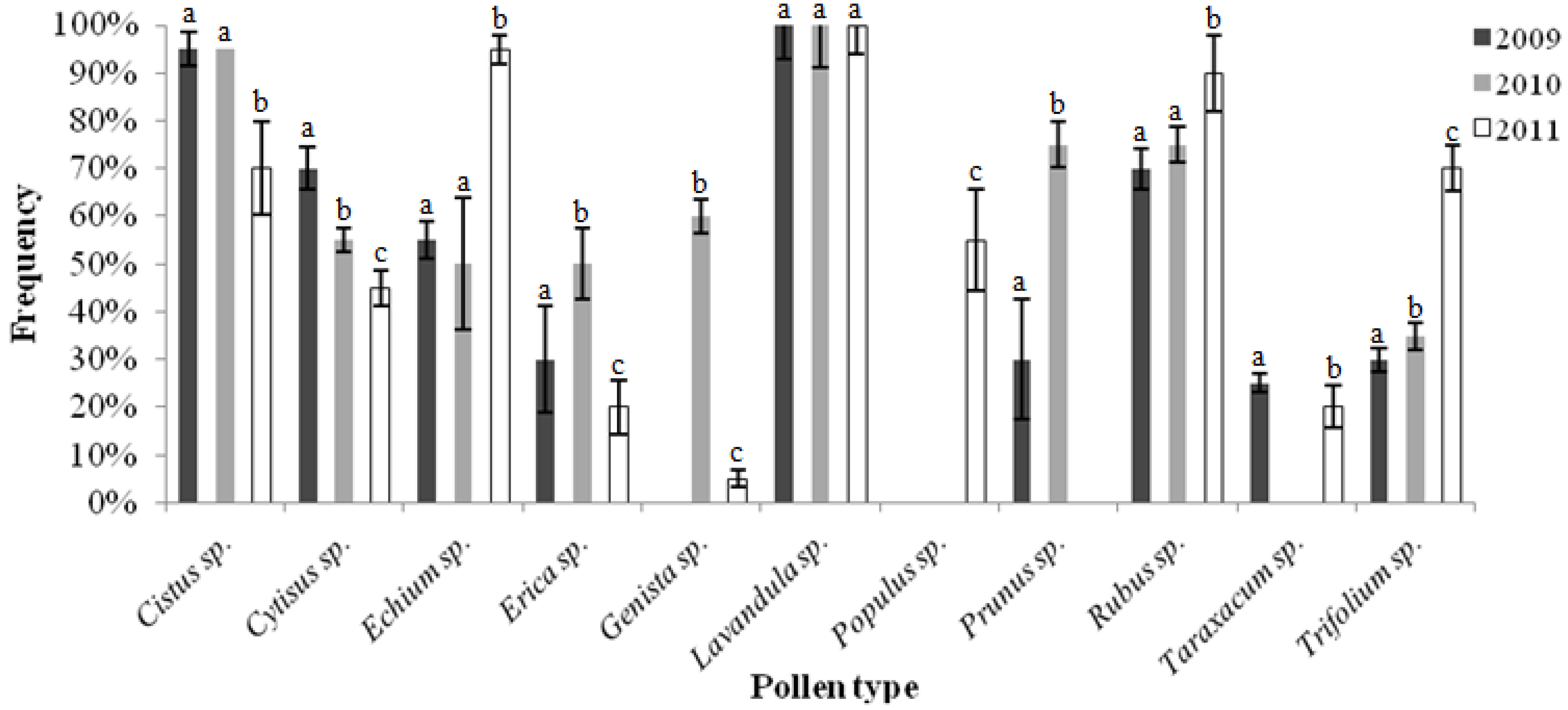
2.2. Colour Analysis
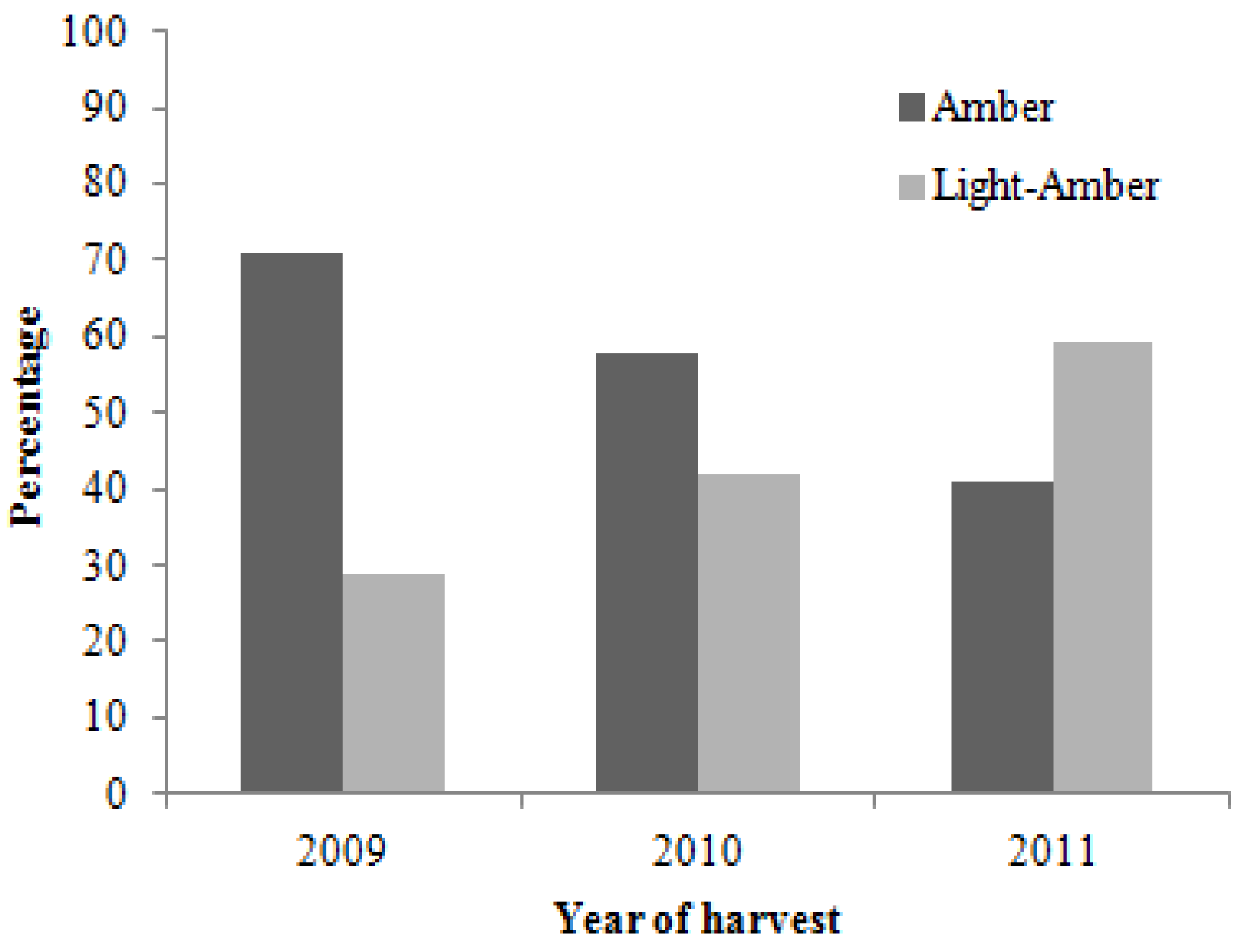
2.3. Physicochemical Analyses
| Harvest (Year) | Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture (%) | Electrical Conductivity(mS·cm−1) | Ash (%) | HMF(mg·kg−1) | Diastase activity(Gothe Scale) | pH | Free acidity(meq·kg−1) | Reducing sugars (%) | Apparent sucrose (%) | ||
| First (2009) | 15.94 ± 0.70 a(14.50–17.21) | 0.25 ± 0.07 b(0.13–0.36) | 0.12 ± 0.06 b(0.02–0.21) | 2.41 ± 1.45 b(1.31–7.02) | 14.04 ± 3.51 b(10.06–25.00) | 3.58 ± 0.14 a(3.32–3.81) | 25.58 ± 6.12 a(13.94–33.63) | 69.75 ± 4.19 a(63.71–76.89) | 4.59 ± 1.51 a(2.60–7.53) | |
| Second (2010) | 16.28 ± 0.46 a(15.40–17.02) | 0.23 ± 0.06 b(0.15–0.36) | 0.10 ± 0.05 b(0.03–0.21) | 4.39 ± 2.64 a(2.63–13.84) | 15.09 ± 1.29 ab(13.59–19.17) | 3.25 ± 0.26 b(2.93–4.12) | 25.16 ± 5.69 a(15.12–38.22) | 66.96 ± 2.91 b(62.52–74.11) | 4.73 ± 1.61 a(2.44–8.12) | |
| Third (2011) | 16.37 ± 0.70 a(15.41–18.11) | 0.29 ± 0.04 a(0.26–0.36) | 0.15 ± 0.03 a(0.12–0.21) | 3.61 ± 1.24 ab(1.71–7.28) | 16.95 ± 2.26 a(11.99–19.10) | 3.28 ± 0.25 b(2.97–3.64) | 28.28 ± 3.93 a(21.52–36.80) | 65.59 ± 2.08 b(60.21–68.57) | 4.54 ± 1.28 a(2.34–7.36) | |
| p-value | 0.082 | 0.006 | <0.001 | 0.006 | 0.002 | <0.001 | 0.143 | <0.001 | 0.914 | |
2.4. Microbiological Analyses
| Harvest | Aerobic mesophiles (cfu·g−1) | Moulds and yeast (cfu·g−1) | Fecal coliforms (MPN) | Sulphite-reducing clostridia (in 0.01 g) | Salmonella (in 25 g) |
|---|---|---|---|---|---|
| First | 38.20 ± 21.62 a | 24.05 ± 11.81 a | <1 | Negative | Negative |
| Second | 37.45 ± 19.99 a | 30.95 ± 22.16 a | <1 | Negative | Negative |
| Third | 37.45 ± 18.27 a | 27.45 ± 18.33 a | <1 | Negative | Negative |
| p-value | 0.681 | 0.482 | n.a. | n.a. | n.a. |
2.5. Multivariate Analysis
| Wilks’ Lambda | Partial Lambda | F-remove(2,45) | p-level | Tolerance | 1-Tolerance(R2) | |
|---|---|---|---|---|---|---|
| Echium sp. | 0.000 | 0.847 | 4.054 | 0.240 | 0.561 | 0.439 |
| Genista sp. | 0.000 | 0.693 | 9.962 | 0.002 | 0.836 | 0.164 |
| Populus sp. | 0.000 | 0.714 | 9.008 | 0.005 | 0.158 | 0.842 |
| Prunus sp. | 0.000 | 0.905 | 2.360 | 0.106 | 0.837 | 0.163 |
| Rubus sp. | 0.000 | 0.962 | 0.898 | 0.415 | 0.476 | 0.524 |
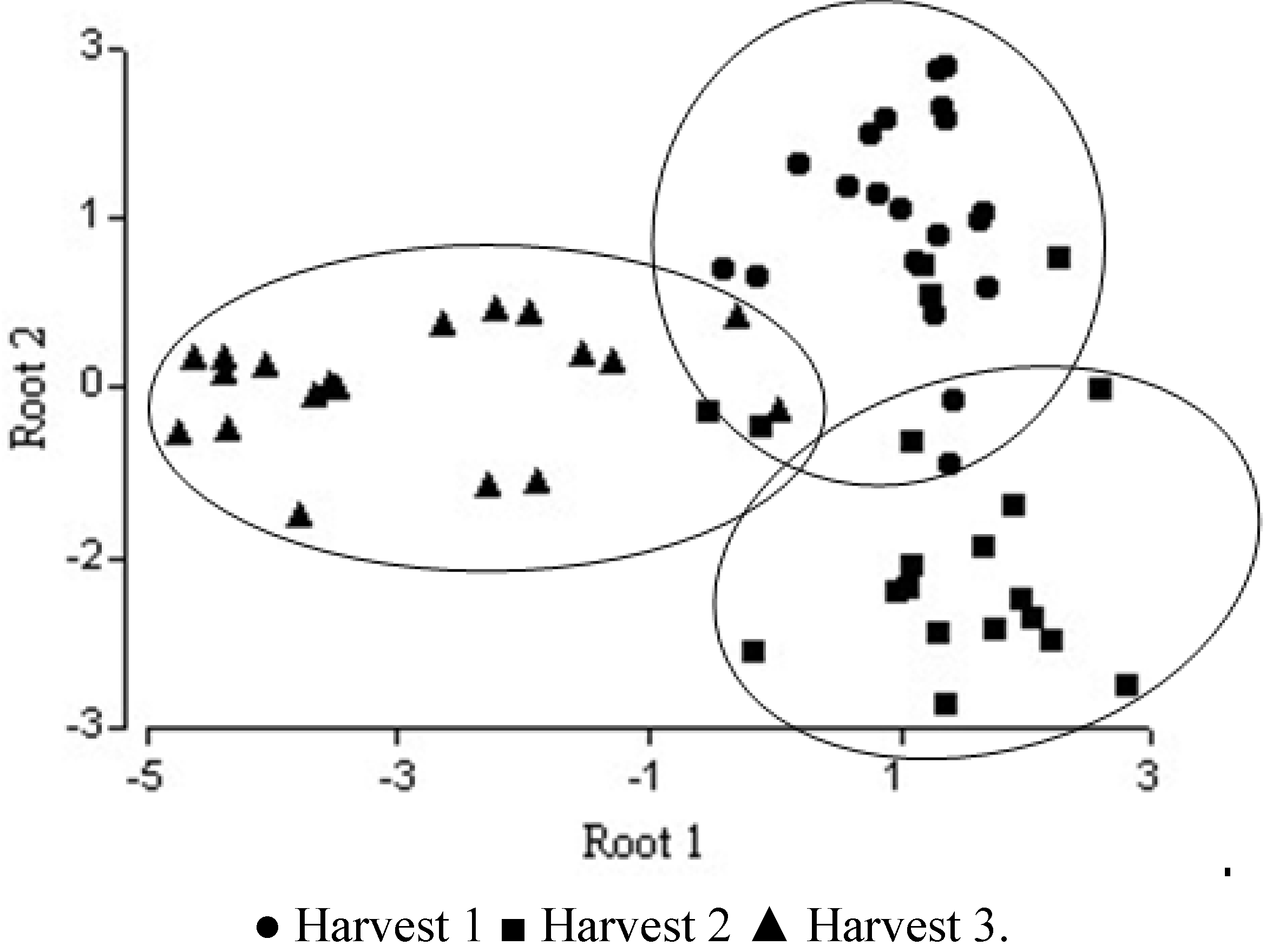
| Wilks’ Lambda | Partial Lambda | F-remove(2,53) | p-level | Tolerance | 1-Tolerance(R2) | |
|---|---|---|---|---|---|---|
| HMF | 0.485 | 0.633 | 15.349 | <0.001 | 0.472 | 0.528 |
| Diastase activity | 0.332 | 0.924 | 2.188 | 0.122 | 0.795 | 0.205 |
| pH | 0.338 | 0.908 | 2.673 | 0.0783 | 0.924 | 0.076 |
| Free acidity | 0.383 | 0.802 | 6.562 | 0.003 | 0.861 | 0.139 |
| Reducing sugars | 0.373 | 0.823 | 5.695 | 0.006 | 0.466 | 0.534 |
3. Experimental
3.1. Chemicals and Materials

3.2. Honey Samples
3.3. Pollen Analyses
3.4. Colour Analyses

3.5. Physicochemical Analyses
3.5.1. Moisture Content
3.5.2. Electrical Conductivity
3.5.3. Ash Content

3.5.4. pH
3.5.5. Free Acidity
3.5.6. Reducing Sugars and Apparent Sucrose
3.5.7. Hydroxymethylfurfural (HMF)

3.5.8. Diastase Activity
3.6. Microbiological Determinations
3.6.1. Sample Preparation
3.6.2. Enumeration of the Total Mesophilic Microorganisms
3.6.3. Enumeration of Yeast and Moulds
3.6.4. Sulphite Reducing Clostridium Spores
3.6.5. Fecal Coliforms and Escherichia Coli
3.6.6. Salmonella
3.6.7. Staphylococcus Aureus
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
References and Notes
- EU, Council Directive 2001/110/CE relating to honey. In Off. J. Eur. Commun.; 2002; L10, pp. 47–52.
- Codex Alimentarius Commission, Revised Codex Standard for Honey; Codex STAN, 12-1981, Rev. 1 1987, Rev. 2. 2001.
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Oligosaccharides might contribute to the antidiabetic effect of honey: A review of the Literature. Molecules 2012, 17, 248–266. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar]
- Küçük, M.; Kolailı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and microbiological effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Fructose might contribute to the hypoglycemic effect of honey. Molecules 2012, 17, 1900–1915. [Google Scholar]
- Ministério da Agricultura do Desenvolvimento Rural e das Pescas. PAN-Programa Apicola Nacional, Trienio de 2011–2013.
- Council Regulation (EC) No 509/2006 of 20 March 2006 on agricultural products and foodstuffs as traditional specialities guaranteed (OJ L 93, 31.3.2006).
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Azeredo, L.C.; Azeredo, M.A.A.; Sousa, S.R.; Dutra, V.M.L. Protein contents and physicochemical properties in honeys sample from Apis mellifera of different floral origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
- Terrab, A.; Díez, M.J.; Heredia, F.J. Characterisation of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002, 79, 373–379. [Google Scholar] [CrossRef]
- Pires, J.; Estevinho, M.L.; Feás, X.; Cantalapiedra, J.; Iglesias, A. Pollen spectrum and physico-chemical attributes of heather (Erica sp.) honeys of north Portugal. J. Sci. Food Agric. 2009, 89, 1862–1870. [Google Scholar]
- Finola, M.S.; Lasagno, M.C.; Marioli, J.M. Microbiological and chemical characterization of honeys from central Argentina. Food Chem. 2007, 100, 1649–1653. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.; Moreira, L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2009, 48, 544–548. [Google Scholar]
- Estevinho, M.L.; Feas, X.; Seijas, J.A.; Vazquez-Tato, M.P. Organic honey from Trás-Os Montes region (Portugal): Chemical, palynological, microbiological and bioactive compounds characterization. Food Chem. Toxicol. 2012, 50, 258–264. [Google Scholar]
- Gomes, T.; Feás, X.; Iglesias, A.; Estevinho, L.M. Study of organic honey from the Northeast of Portugal. Molecules 2011, 16, 5374–5386. [Google Scholar]
- Giorgi, A.; Madeo, M.; Baumgartner, J.; Lozzia, G.C. The relationships between Phenolic content, Pollen diversity, physicochemical information and radical scavenging activity in honey. Molecules 2011, 16, 336–347. [Google Scholar]
- Nevas, M.; Lindstrom, M.; Hautamaki, K.; Puoskari, S.; Korkeala, H. Prevalence and diversity of Clostridium botulinum types A, B, E and F in honey produced in the Nordic countries. Int. J. Food Microbiol. 2005, 105, 145–151. [Google Scholar] [CrossRef]
- Cunha, A.P.; Ribeiro, J.A.; Roque, O.R. Plantas aromáticas em Portugal. Caracterização e Utilizações; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2007. [Google Scholar]
- Olaitan, P.B.; Adeleke, O.E.; Ola, I.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physicochemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem. 2007, 101, 695–703. [Google Scholar] [CrossRef]
- Tosi, E.; Martinet, R.; Ortega, M.; Lucero, H.; Ré, E. Honey diastase activity modified by heating. Food Chem. 2008, 106, 883–887. [Google Scholar] [CrossRef]
- Fallico, B.; Arena, E.; Verzera, A.; Zappala, M. The European food legislation and its impact on honey sector. Accredit. Qual. Assur. 2006, 11, 49–54. [Google Scholar] [CrossRef]
- Rodriguez, G.O.; Ferrer, B.S.; Ferrer, A.; Rodriguez, B. Characterization of honey produced in Venezuela. Food Chem. 2004, 84, 499–502. [Google Scholar] [CrossRef]
- Persano-Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Andrade, P.B.; Amaral, M.T.; Isabel, P.; Carvalho, J.C.M.F.; Seabra, R.M.; Proença da Cunha, A. Physicochemical attributes and pollen spectrum of Portuguese heather honeys. Food Chem. 1999, 66, 503–510. [Google Scholar]
- Sodré, G.S.; Marchini, L.C.; Moreti, A.C.C. Caracterização físico-química de amostras de méis de Apis mellifera L. (Hymenoptera: Apidae) do Estado do Ceará. Cienc. Rur. 2007, 37, 1139–1144. [Google Scholar]
- Iurlina, M.O.; Fritz, R. Characterization of microorganisms in Argentinean honeys from different sources. Int. J. Food Microbiol. 2005, 105, 297–304. [Google Scholar] [CrossRef]
- Feás, X.; Pires, J.; Iglesias, A.; Estevinho, M.L. Characterization of artisanal honey produced on the Northwest of Portugal by melissopalynological and physico-chemical data. Food Chem. Toxicol. 2010, 48, 3462–3470. [Google Scholar] [CrossRef]
- Feás, X.; Pires, J.; Estevinho, M.L.; Iglesias, A.; Pinto de Araujo, J.P. Palynological and physicochemical data characterisation of honeys produced in the Entre-Douro e Minho region of Portugal. Int. J. Food Sci. Tech. 2010, 45, 1255–1262. [Google Scholar]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, M.L. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar]
- Association of Official Analytical Chemists (AOAC), Official Methods of Analysis, 15th ed; AOAC: Arlington, TX, USA, 1990.
- Bogdanov, S.; Martin, P.; Lüllman, C.; Borneck, R.; Morlot, M.; Heritier, J.; Vorwohl, G.; Russmann, H.; Persano-Oddo, L.; Sabatini, A.G.; et al. Harmonised Methods of The European Honey Commission. Apidologie 1997, 28, 1–59. [Google Scholar]
- Sancho, M.T.; Muniategui, S.; Sánchez, M.P.; Huidobro, J.F.; Simal, J. Relationships between electrical conductivity and total and sulphated ash contents in Basque honeys. Apidologie 1991, 22, 487–494. [Google Scholar] [CrossRef]
- Sample Availability: Samples of honey are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iglesias, A.; Feás, X.; Rodrigues, S.; Seijas, J.A.; Vázquez-Tato, M.P.; Dias, L.G.; Estevinho, L.M. Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications. Molecules 2012, 17, 8561-8577. https://doi.org/10.3390/molecules17078561
Iglesias A, Feás X, Rodrigues S, Seijas JA, Vázquez-Tato MP, Dias LG, Estevinho LM. Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications. Molecules. 2012; 17(7):8561-8577. https://doi.org/10.3390/molecules17078561
Chicago/Turabian StyleIglesias, Antonio, Xesus Feás, Sandra Rodrigues, Julio A. Seijas, M. Pilar Vázquez-Tato, Luís G. Dias, and Leticia M. Estevinho. 2012. "Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications" Molecules 17, no. 7: 8561-8577. https://doi.org/10.3390/molecules17078561
APA StyleIglesias, A., Feás, X., Rodrigues, S., Seijas, J. A., Vázquez-Tato, M. P., Dias, L. G., & Estevinho, L. M. (2012). Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications. Molecules, 17(7), 8561-8577. https://doi.org/10.3390/molecules17078561










