Two New Oxysporone Derivatives from the Fermentation Broth of the Endophytic Plant Fungus Pestalotiopsis karstenii Isolated from Stems of Camellia sasanqua
Abstract
:1. Introduction
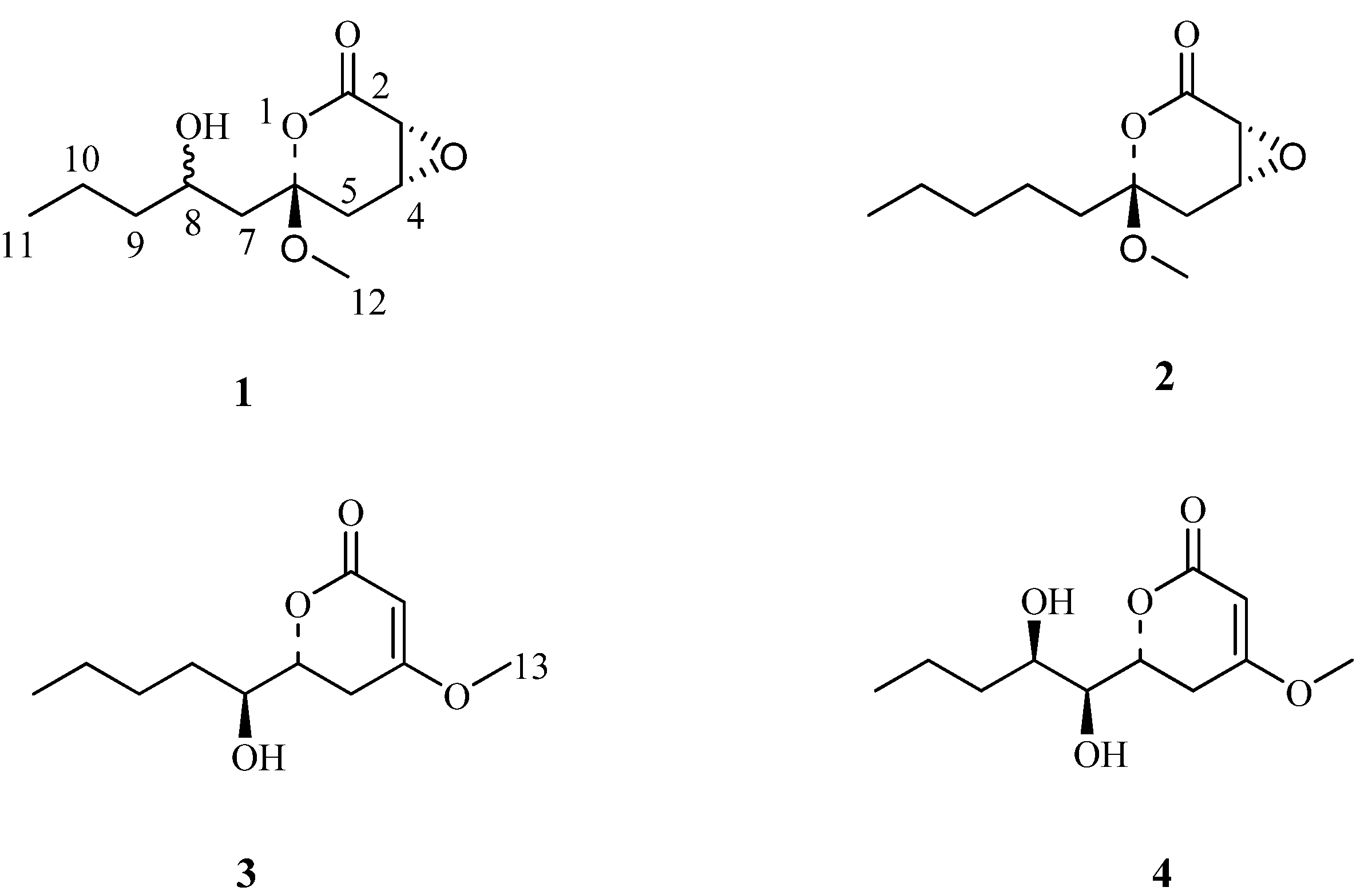
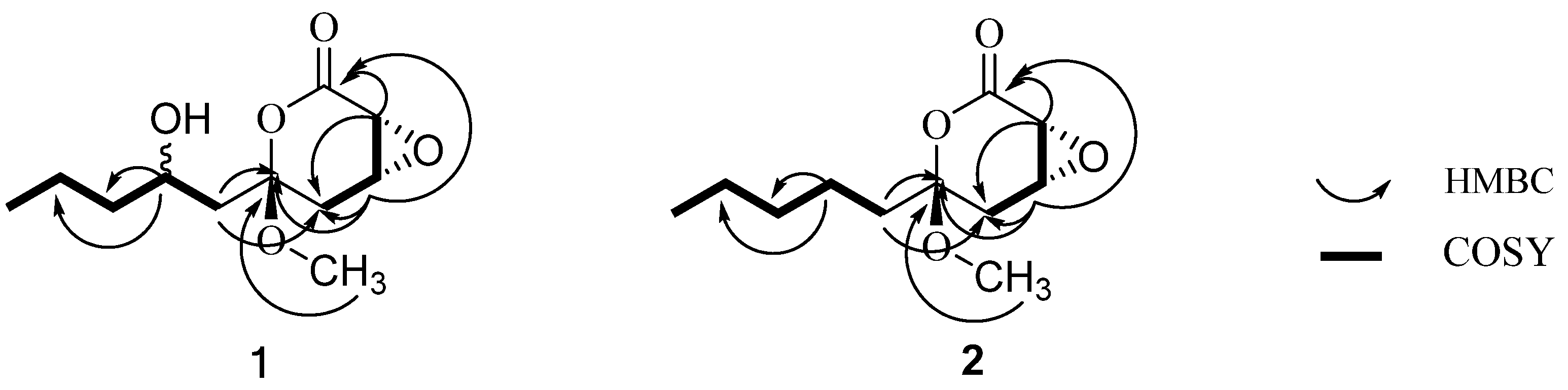
2. Results and Discussion
| NO. | Pestalrone A (1) | Pestalrone B (2) | ||
|---|---|---|---|---|
| δH a ( J in Hz) | δC b | δH a ( J in Hz) | δC b | |
| 2 | 169.2 (s) | 169.0 (s) | ||
| 3 | 3.65 (1H, d, J = 2.8) | 76.4 (d) | 4.17 (1H, d, 2.1) | 83.1 (d) |
| 4 | 4.82 (1H, dt, J = 4.0, 2.3) | 71.7 (d) | 4.80 (1H, t, J = 3.0) | 78.8 (d) |
| 5 | 1.88 (1H, dd,
J = 13.7, 4.1) 2.42 (1H, m) | 29.1 (t) | 2.03 (1H, d,
J = 12.5) 2.48 (1H, dt, 12.5, 2.9) | 35.8 (t) |
| 6 | 95.7 (s) | 104.7 (s) | ||
| 7 | 2.78 (1H, d,
J = 18.6) 2.98 (1H, dd, J = 18.6, 2.7) | 40.7 (t) | 2.81 (1H, d,
J = 17.9) 3.03 (1H, dd, J = 17.9, 2.1) | 45.7 (t) |
| 8 | 3.67 (1H, m) | 66.2 (d) | 1.61 (2H, m) | 27.5 (t) |
| 9 | 1.73 (1H, m) 1.39 (1H, m) | 32.5 (t) | 1.37 (2H, m) | 30.5 (t) |
| 10 | 1.51 (2H, m) | 18.6 (t) | 1.37 (2H, m) | 22.6 (t) |
| 11 | 0.96 (3H, t, J = 7.2) | 14.0 (q) | 0.92 (3H, m) | 13.9 (q) |
| 12 | 3.39 (3H, s) | 48.9 (q) | 3.43 (3H, s) | 50.0 (q) |
3. Experimental
3.1. General
3.2. Fungal Material and Cultivation Conditions
3.3. Extraction and Isolation
3.4. Biological Assays
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Chauhan, S. A new species of the genus Pestalotiopsis from Indian soil. Indian Phytopathol. 1988, 41, 625–627. [Google Scholar]
- Ding, G.; Qi, Y.; Liu, S.L.; Chen, G.X. Photipyrones A and B, New pyrone derivatives from the plant endophytic fungus Pestalotiopsis photiniae. J. Antibiot. 2012, 65, 271–273. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, J.Z.; Luo, D.Q. The taxonomy, Biology and chemistry of the fungal Pestalotiopsis genus. Nat. Prod. Rep. 2012, 29, 622–641. [Google Scholar]
- Li, J.; Wu, X.F.; Ding, G.; Feng, Y.; Jiang, X.J.; Guo, L.D.; Che, Y.S. α-Pyrones and pyranes from the plant pathogenicfungus Pestalotiopsis scirpina. Eur. J. Org. Chem. 2012, 2445–2452. [Google Scholar]
- Li, J.; Li, L.; Si, Y.; Jiang, X.L.; Che, G.Y. Virgatolides A-C, Benzannulated spiroketals from the plant endophytic fungus Pestalotiopsis virgatula. Org. Lett. 2011, 13, 2670–2673. [Google Scholar]
- Yang, X.L.; Zhang, S. Dihydroberkleasmin A: A New Eremophilane Sesquiterpenoid from the fermentation broth of the plant endophytic fungus Pestalotiopsis photiniae. Molecules 2011, 16, 1910–1916. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, S. Eremophilane-type Sesquiterpenoids from the fermentation broth of plant endophytic fungus Pestalotiopsis photiniae isolated from the Chinese podocarpaceae plant Podocarpus macrophyllus. Helv. Chim. Acta 2011, 94, 1463–1468. [Google Scholar] [CrossRef]
- Luo, D.Q.; Deng, H.Y. Oleanane-type triterpenoids from endophytic fungus Pestalotiopsis clavispora isolated from the Chinese mangrove plant Bruguiera sexangula. Helv. Chim. Acta 2011, 94, 1041–1047. [Google Scholar] [CrossRef]
- Ding, G.; Zhang, F. Pestaloquinols A and B, isoprenylated epoxyquinols from Pestalotiopsis sp. J. Nat. Prod. 2011, 74, 286–291. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R. Pestalactams A–C: Novel caprolactams from the endophytic fungus Pestalotiopsis sp. Org. Biomol. Chem. 2010, 8, 1785–1790. [Google Scholar] [CrossRef]
- Fu, S.B.; Yang, J.S. Multihydroxylation of ursolic acid by Pestalotiopsis microspora isolated from the medicinal plant Huperzia serrata. Fitoterapia 2011, 82, 1057–1061. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.B. Pestalotiopamide E, A new amide from the endophytic fungus Pestalotiopsis sp. J. Asian Nat. Prod. Res. 2011, 13, 373–376. [Google Scholar] [CrossRef]
- Li, J.; Li, L. Virgatolides A–C, benzannulated spiroketals from the plant endophytic fungus Pestalotiopsis virgatula. Org. Lett. 2011, 13, 2670–2673. [Google Scholar] [CrossRef]
- Strobel, G.A.; Hess, W.M. Taxol from fungal endophytes and the issue of biodiversity. J. Ind. Microbiol. 1996, 17, 417–423. [Google Scholar] [CrossRef]
- Li, J.Y.; Strobel, G.A. Endophytic taxol-producing fungi from bald cypress, Taxodium distichum. Microbiology 1996, 142, 2223–2226. [Google Scholar] [CrossRef]
- Kimura, Y.; Hamasaki, T. Sterechemistry and Biological Activities of LL-P880γ, a Pestalotin Analogue, Produced by Penicillium citreo-viride. Agric. Biol. Chem. 1986, 50, 1649–1650. [Google Scholar] [CrossRef]
- McGahren, W.J.; Ellestad, G.A. A new fungal lactone, LL-P880 beta, and a new pyrone, LL-P880 gamma, from a Penicillium sp. J. Org. Chem. 1973, 38, 3542–3544. [Google Scholar] [CrossRef]
- Mosmman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Zhou, J.J.; Yue, X.F. Improved MTT assay for acofantitumor agents. Chin. J. Pharm. 1993, 24, 455–457. [Google Scholar]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luo, D.Q.; Zhang, L.; Shi, B.Z.; Song, X.M. Two New Oxysporone Derivatives from the Fermentation Broth of the Endophytic Plant Fungus Pestalotiopsis karstenii Isolated from Stems of Camellia sasanqua. Molecules 2012, 17, 8554-8560. https://doi.org/10.3390/molecules17078554
Luo DQ, Zhang L, Shi BZ, Song XM. Two New Oxysporone Derivatives from the Fermentation Broth of the Endophytic Plant Fungus Pestalotiopsis karstenii Isolated from Stems of Camellia sasanqua. Molecules. 2012; 17(7):8554-8560. https://doi.org/10.3390/molecules17078554
Chicago/Turabian StyleLuo, Du Qiang, Lei Zhang, Bao Zhong Shi, and Xiao Mei Song. 2012. "Two New Oxysporone Derivatives from the Fermentation Broth of the Endophytic Plant Fungus Pestalotiopsis karstenii Isolated from Stems of Camellia sasanqua" Molecules 17, no. 7: 8554-8560. https://doi.org/10.3390/molecules17078554
APA StyleLuo, D. Q., Zhang, L., Shi, B. Z., & Song, X. M. (2012). Two New Oxysporone Derivatives from the Fermentation Broth of the Endophytic Plant Fungus Pestalotiopsis karstenii Isolated from Stems of Camellia sasanqua. Molecules, 17(7), 8554-8560. https://doi.org/10.3390/molecules17078554




