Influence of ShuJinHuoXue Tablets on Ischemia Reperfusion Injury of Animals’ Skeletal Muscle
Abstract
:1. Introduction
2. Results and Discussion
| Group | NO (μmol/L) | ET-1 (ng/L) | NO/ET-1 | TXB2 (ng/L) | 6-Keto-PGF1α (ng/L) | TXB2/ 6-Keto-PGF1α |
|---|---|---|---|---|---|---|
| NC | 31.67 ± 2.99 | 79.53 ± 6.81 | 0.39 ± 0.03 | 201.43 ± 18.57 | 659.03 ± 57.77 | 0.316 ± 0.028 |
| IR | 50.82 ± 4.82 b | 275.83 ± 24.47 b | 0.18 ± 0.01 b | 517.39 ± 49.05 b | 871.62 ± 80.32 b | 0.594 ± 0.063 b |
| IR+SJHXT (200 mg/kg/day) | 59.09 ± 6.48 | 227.09 ± 22.85 d | 0.26 ± 0.02 c | 487.27 ± 44.13 c | 968.26 ± 90.49 d | 0.503 ± 0.047 c |
| IR+SJHXT (300 mg/kg/day) | 66.48 ± 6.03 d | 197.53 ± 21.68 d | 0.34 ± 0.03 d | 451.38 ± 48.01 d | 1083.44 ± 93.68 d | 0.416 ± 0.038 d |
| IR+SJHXT (400 mg/kg/day) | 71.24 ± 8.01 d | 152.69 ± 17.05 d | 0.47 ± 0.04 d | 406.14 ± 37.84 d | 1192.49 ± 121.07 d | 0.341 ± 0.033 d |
| Group | CK (μkat/L) | LDH (μkat/L) |
|---|---|---|
| NC | 93.06 ± 11.76 | 6.37 ± 0.77 |
| IR | 424.57 ± 52.87 b | 22.81 ± 2.41 b |
| IR+SJHXT (200 mg/kg/day) | 328.06 ± 47.18 d | 16.77 ± 2.05 d |
| IR+SJHXT (300 mg/kg/day) | 247.11 ± 36.01 d | 11.59 ± 1.31 d |
| IR+SJHXT (400 mg/kg/day) | 169.03 ± 18.09 d | 8.04 ± 0.92 d |
| Group | MPO (μkat/L) | W/D |
|---|---|---|
| NC | 1.46 ± 0.18 | 5.01 ± 0.48 |
| IR | 4.39 ± 0.51 b | 6.94 ± 0.66 b |
| IR+SJHXT (200 mg/kg/day) | 3.88 ± 0.42 c | 6.43 ± 0.59 |
| IR+SJHXT (300 mg/kg/day) | 3.05 ± 0.29 d | 5.92 ± 0.55 c |
| IR+SJHXT (400 mg/kg/day) | 2.41 ± 0.22 d | 5.61 ± 0.48 c |
| Group | Ca2+ (mmol/g prot) | Na+-K+-ATPase (μmol Pi/mg prot/hour) | Ca2+-Mg2+-ATPase (μmol Pi/mg prot/hour) |
|---|---|---|---|
| NC | 0.062 ± 0.007 | 2.38 ± 0.27 | 2.57 ± 0.31 |
| IR | 0.191 ± 0.013 b | 1.27 ± 0.14 b | 1.39 ± 0.15 b |
| IR+SJHXT (200 mg/kg/day) | 0.145 ± 0.011 c | 1.61 ± 0.19 | 1.75 ± 0.19 c |
| IR+SJHXT (300 mg/kg/day) | 0.107 ± 0.012 d | 1.93 ± 0.21 c | 2.28 ± 0.25 d |
| IR+SJHXT (400 mg/kg/day) | 0.082 ± 0.007 d | 2.22 ± 0.24 d | 2.49 ± 0.26 d |
| Group | MDA (nmol/mg protein) | SOD (U/mg protein) | CAT (U/mg protein) | GSH-Px (U/mg protein) |
|---|---|---|---|---|
| NC | 3.16 ± 0.34 | 304.7 ± 34.15 | 68.31 ± 7.16 | 73.09 ± 6.88 |
| IR | 7.92 ± 0.68 b | 168.3 ± 18.59 b | 35.05 ± 4.03 b | 30.61 ± 3.71 b |
| IR+SJHXT (200 mg/kg/day) | 6.24 ± 0.66 d | 199.4 ± 22.11 d | 47.41 ± 5.12 d | 48.92 ± 5.05 d |
| IR+SJHXT (300 mg/kg/day) | 5.02 ± 0.57 d | 264.8 ± 30.08 d | 57.07 ± 6.06 d | 60.51 ± 7.82 d |
| IR+SJHXT (400 mg/kg/day) | 4.28 ± 0.51 d | 290.4 ± 31.49 d | 66.17 ± 7.29 d | 79.03 ± 6.79 d |
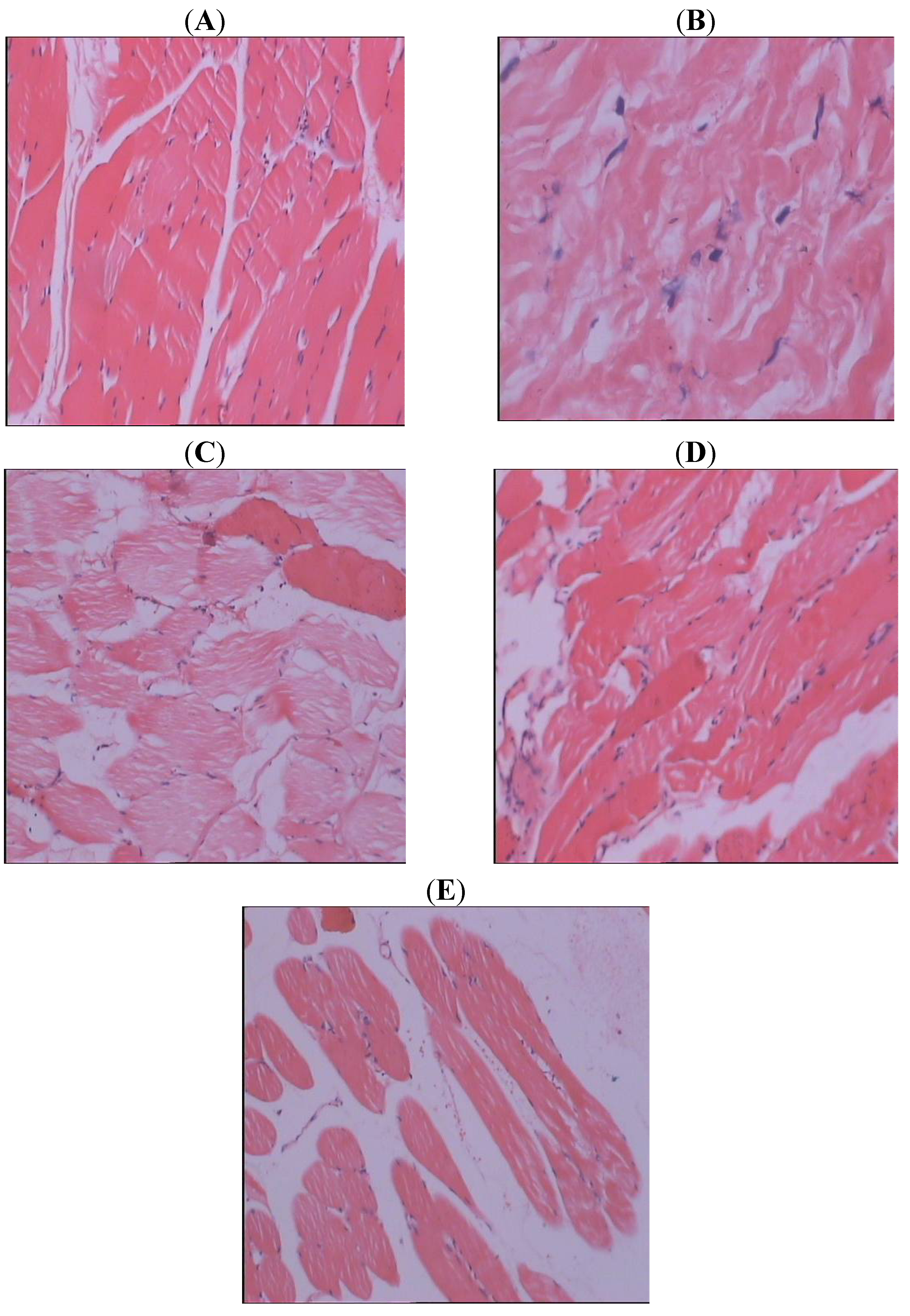
3. Experimental
3.1. Materials
3.2. Animals
3.3. Biochemical Assays
3.4. Wet Weight/Dried Weight Assay
3.5. Histopathological Study
3.6. Statistics
4. Conclusion
References
- Cuzzocrea, S.; Ridley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation and ischemia/reperfusion injury. Pharmacol. Rev. 2001, 53, 135–159. [Google Scholar]
- Anaya-Prado, R.; Toledo-Pereya, L.H.; Lentsch, A.B.; Ward, P.A. Ischemia/reperfusion injury. J. Surg. Res. 2002, 105, 248–258. [Google Scholar]
- Heunks, L.M.A.; Dekhuijzen, P.N.R. Respiratory muscle function and free radicals: From cell to COPD. Thorax 2000, 55, 704–716. [Google Scholar] [CrossRef]
- Pattwell, D.; Mcardlle, A.; GriVths, R.D.; Jackson, M.J. Measurement of free radical production by in vivo microdialysis during ischemia/reperfusion injury to skeletal muscle. Free Radic. Biol. Med. 2000, 30, 979–985. [Google Scholar]
- Hallström, S.; Gasser, H.; Neumayer, C.; Fugl, A.; Nanobashvili, J.; Jakubowski, A.; Huk, I.; Schlag, G.; Malinski, T. S-Nitroso human serum albumin treatment reduces ischemia/reperfusion injury in skeletal muscle via nitric oxide release. Circulation 2002, 105, 3032–3038. [Google Scholar] [CrossRef]
- Nakamura, K.; Yokoyama, K.; Nakamura, K.; Itoman, M. Changes in nitric oxide, superoxide, and blood circulation in muscles over time after warm ischaemic reperfusion in rabbit rectus femoris muscle. Scand. J. Plast. Reconstr. Hand Surg. 2001, 35, 13–18. [Google Scholar]
- Khanna, A.; Cowled, P.; Fitridge, R.A. Nitric oxide and skeletal muscle reperfusion injury: Current controversies (research review). J. Surg. Res. 2005, 128, 98–107. [Google Scholar]
- Beyersdorf, F. Protection of the ischemic skeletal muscle. Thorac Cardiovasc. Surg. 1991, 39, 19–28. [Google Scholar] [CrossRef]
- Faust, K.B.; Chiantella, V.; Johansen-Vinten, J.; Meredith, J.H. Oxygen-derived free radical scavengers and skeletal muscle ischemic/reperfusion injury. Am. Surg. 1988, 54, 709–719. [Google Scholar]
- Feller, A.M.; Roth, C.A.; Russel, C.R.; Eagleton, B.; Suchy, N.; Debs, N. Experimental evaluation of oxygen free radicals scavengers in the prevention of reperfusion injury skeletal muscle. Ann. Plastic. Surg. 1989, 22, 321–330. [Google Scholar] [CrossRef]
- Haimovici, H. Ischemia-reperfusion syndrome of skeletal muscle. J. Cardiovasc. Surg. 1990, 31, 318–319. [Google Scholar]
- Korthius, J.R.; Granger, N.D.; Townsley, M.I.; Taylor, A.E. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ. Res. 1985, 57, 599–609. [Google Scholar] [CrossRef]
- Saez, C.J.; Cifuentes, F.; Wards, H.P.; Gunther, B.; Vivaldi, E. Tourniquet shock in rats: Effects of allopurinol on biochemical changes of the gastrocnemius muscle subjected to ischemia followed by reperfusion. Biochem. Med. Met. Biol. 1986, 35, 199–209. [Google Scholar] [CrossRef]
- Kerrigan, C.L.; Stotland, M.A. Ischemia reperfusion injury: A review. Microsurgery 1993, 14, 165–175. [Google Scholar] [CrossRef]
- Schlag, M.G.; Harris, K.A.; Potter, R.F. Role of leukocyte accumulation and oxygen radicals in ischemia-reperfusion-induced injury in skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, 1716–1721. [Google Scholar]
- Blaisdell, F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc. Surg. 2002, 10, 620–630. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Arumugam, T.V.; Shiels, I.A. Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J. Surg. Res. 2004, 116, 81–90. [Google Scholar] [CrossRef]
- Malinski, T.; Bailey, F.; Zhang, Z.G.; Chopp, M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J. Cereb. Blood Flow. Metab. 1993, 13, 355–358. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, P.L.; Panahian, N.; Dalkara, T.; Fishman, M.C.; Moskowitz, M.A. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994, 265, 1883–1885. [Google Scholar]
- Ohta, K.; Graf, R.; Rosner, G.; Kumura, E.; Heiss, W.D. Profiles of cortical tissue depolarization in cat focal cerebral ischemia in relation to calcium ion homeostasis and nitric oxide production. J. Cereb. Blood Flow Metab. 1997, 17, 1170–1181. [Google Scholar]
- Jiang, M.H.; Kaku, T.; Hada, J.; Hayashi, Y. 7-Nitroindazole reduces nitric oxide concentration in rat hippocampus after transient forebrain ischemia. Eur. J. Pharmacol. 1999, 380, 117–121. [Google Scholar] [CrossRef]
- Christo, J.S.; Rodrigues, A.M.; Mouro, M.G.; Cenedeze, M.A.; de Jesus Simões, M.; Schor, N.; Higa, E.M.S. Nitric oxide (NO) is associated with gentamicin (GENTA) nephrotoxicity and the renal function recovery after suspension of GENTA treatment in rats. Nitric Oxide 2011, 24, 77–83. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Roux, S.; Breu, V.; Ertel, S.I.; Clozel, M. Endothelin antagonism with bosentan: A review of potential applications. J. Mol. Med. 1999, 77, 364–376. [Google Scholar] [CrossRef]
- Jo, S.M.; Ryu, H.J.; Kim, J.E.; Yeo, S.I.; Kim, M.J.; Choi, H.C.; Song, H.K.; Kang, T.C. Up-Regulation of endothelial endothelin-1 expression prior to vasogenic edema formation in the rat piriform cortex following status epilepticus. Neurosci. Lett. 2011, 501, 25–30. [Google Scholar] [CrossRef]
- Tay, S.K.; Ong, H.T.; Low, P.S. Transaminitis in Duchenne’s muscular dystrophy. Ann. Acad. Med. Singapore 2000, 29, 719–722. [Google Scholar]
- Carter, W.O.; Bull, C.; Bortolon, E.; Yang, L.; Jesmok, G.J.; Gundel, R.H. A murine skeletal muscle ischemiareperfusion injury model: Differential pathology in BALB/c and DBA/2N mice. J. Appl. Physiol. 1998, 85, 1676–1683. [Google Scholar]
- Papanastasiou, S.; Estdale, S.E.; Homer-Vanniasinkam, S.; Mathie, R.T. Protective effect of preconditioning and adenosine pretreatment in experimental skeletal muscle reperfusion injury. Br. J. Surg. 1999, 86, 916–922. [Google Scholar] [CrossRef]
- Smith, J.K.; Grisham, M.B.; Granger, D.N.; Korthuis, R.J. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am. J. Physiol. 1989, 256, 789–793. [Google Scholar]
- Aminian, A.; Boudjeltia, K.Z.; Babar, S.; Antwerpen, P.V.; Lefebvre, P.; Crasset, V.; Leone, A.; Ducobu, J.; Friart, A.; Vanhaeverbeek, M. Coronary stenting is associated with an acute increase in plasma myeloperoxidase in stable angina patients but not in patients with acute myocardial infarction. Eur. J. Int. Med. 2009, 20, 527–532. [Google Scholar]
- Grisham, M.B.; Hernandez, L.A.; Granger, D.N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am. J. Physiol. 1986, 251, 567–574. [Google Scholar]
- Granger, D.N.; Barrowman, J.A. Microcirculation of the alimentary tract. II. Pathophysiology of edema. Gastroenterology 1983, 84, 1035–1049. [Google Scholar]
- Mullane, K.M.; Read, N.; Salmon, J.A.; Moncada, S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: Relationship to myocardial salvage by anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 1984, 228, 510–522. [Google Scholar]
- Romson, J.L.; Hook, B.G.; Kunkel, S.L.; Abrams, G.D.; Schork, M.A.; Lucchesi, B.R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 1983, 67, 1016–1023. [Google Scholar]
- Lee, K.R.; Cronenwett, J.L.; Shlafer, M.; Corpron, C.; Zelenock, G.B. Effect of superoxide dismutase plus catalase on Ca2+ transport in ischemic and reperfused skeletal muscle. J. Surg. Res. 1987, 42, 24–32. [Google Scholar] [CrossRef]
- Jørgensen, P.L.; Nielsen, J.M.; Rasmusssen, J.H.; Pedersen, P.A. Structure-function relationships of E1-E2 transitions and cation binding in Na-K pump protein. Biochem. Biophys. Acta 1998, 1365, 65–70. [Google Scholar] [CrossRef]
- Torben, C. Na+-K+ Pump regulation and skeletal muscle contractility. Physiol. Rev. 2003, 83, 1269–1324. [Google Scholar]
- Shivanna, B.D.; Rowe, E.S. Preservation of the native structure and function of Ca2+-ATPase from sarcoplasmic reticulum: Solubilization and reconstitution by new short—chain phospholipid detergent 1,2-diheptanoyl-sn-phosphatidylcholine. Biochem. J. 1977, 325, 533–542. [Google Scholar]
- Gallo, L.C.; Davel, A.P.C.; Xavier, F.E.; Rossoni, L.V. Time-dependent increases in ouabain-sensitive Na+, K+-ATPase activity in aortas from diabetic rats: The role of prostanoids and protein kinase C. Life Sci. 2010, 87, 302–308. [Google Scholar]
- Belkin, M.; LaMorte, W.L.; Wright, J.G.; Hobson, R.W. The role of leukocytes in the pathophysiology of skeletal muscle ischemic injury. J. Vasc. Surg. 1989, 10, 14–19. [Google Scholar]
- Petrasek, P.F.; Homer-Vanniasinkam, S.; Walker, P.M. Determinants of ischemic injury to skeletal muscle. J. Vasc. Surg. 1994, 19, 623–631. [Google Scholar] [CrossRef]
- Koksal, C.; Bozkurt, A.K.; Cangel, U.; Ustundag, N.; Konukoglu, D.; Musellim, B.; Gurel, A. Attenuation of ischemia/reperfusion injury byN-acetylcysteine in a rat hind limb model. J. Surg. Res. 2003, 111, 236–239. [Google Scholar] [CrossRef]
- Tuncel, N.; Erden, S.; Uzuner, K.; Altýokka, G.; Tuncel, M. Ischemic-reperfused rat skeletal muscle: The effect of vasoactive intestinal peptide (VIP) on contractile force, oxygenation and antioxidant enzyme systems. Peptides 1997, 18, 269–275. [Google Scholar]
- Belkin, M.; LaMorte, W.L.; Wright, J.G.; Hobson, R.W., II. The role of leukocytes in the pathophysiology of skeletal muscle ischemic injury. J. Vasc. Surg. 1989, 10, 14–18. [Google Scholar]
- Petrasek, P.F.; Homer-Vanniasinkam, S.; Walker, P.M. Determinants of ischemic injury to skeletal muscle. J. Vasc. Surg. 1994, 19, 623–631. [Google Scholar] [CrossRef]
- Avci, G.; Kadioglu, H.; Sehirli, A.O.; Bozkurt, S.; Guclu, O.; Arslan, E.; Muratli, S.K. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J. Surg. Res. 2012, 172, 39–46. [Google Scholar] [CrossRef]
- Bozkurt, A.; Cakir, B.; Ercan, F.; Yegen, B.C. Anti-inflammatory effects of leptin and cholecystokinin on acetic acid-induced colitis in rats: Role of capsaicin-sensitive vagal afferent fibers. Regul. Pept. 2003, 116, 109–118. [Google Scholar]
- Gerbi, A.; Zérouga, M.; Maixent, J.-M.; Debray, M.; Durand, G.; Bourre, J.M. Diet deficient in alpha-linolenic acid alters fatty acid composition and enzymatic properties of Na+, K+-ATPase isoenzymes of brain membranes in the adult rat. J. Nutr. Biochem. 1999, 10, 230–236. [Google Scholar] [CrossRef]
- Yoshioka, T.; Tanaka, O. Histochemical localization of Ca2+, Mg2+-ATPase of the rat cerebellar cortex during postnatal development. Int. J. Dev. Neurosci. 1989, 7, 181–193. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar]
- Jain, S.K.; McVie, R.; Duett, J.; Herbst, J.J. Erythrocyte membrane lipid peroxidation and glycolylated hemoglobin in diabetes. Diabetes 1989, 38, 1539–1543. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Bergmeyer, H.U., Gawehn, K., Eds.; Academic Press: New York, NY, USA, 1974; Volume 2, pp. 673–884. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Comm. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Sengottuvelan, M.; Viswanathan, P.; Nalini, N. Chemopreventive effect of trans-resveratrol - a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis 2006, 27, 1038–1046. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the ShuJinHuoXue tablet are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tong, Z.; Yu, F.; Liu, Z.; Liang, H. Influence of ShuJinHuoXue Tablets on Ischemia Reperfusion Injury of Animals’ Skeletal Muscle. Molecules 2012, 17, 8494-8505. https://doi.org/10.3390/molecules17078494
Tong Z, Yu F, Liu Z, Liang H. Influence of ShuJinHuoXue Tablets on Ischemia Reperfusion Injury of Animals’ Skeletal Muscle. Molecules. 2012; 17(7):8494-8505. https://doi.org/10.3390/molecules17078494
Chicago/Turabian StyleTong, Zhihong, Fang Yu, Zhonghua Liu, and Haidong Liang. 2012. "Influence of ShuJinHuoXue Tablets on Ischemia Reperfusion Injury of Animals’ Skeletal Muscle" Molecules 17, no. 7: 8494-8505. https://doi.org/10.3390/molecules17078494




