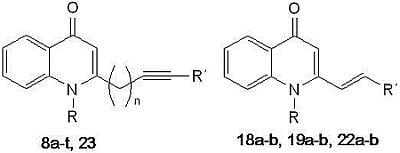
3.2.9. General Procedure for the Synthesis of 8a–t, 18a–b, 19a–b, 22a–b and 23
Compounds
8a–t,
18a–b,
19a–b,
22a–b and
23 were prepared according to procedure described previously [
10] from methyl alkynyl ketones (1.0 equiv.) in THF, LDA (1.8 M in THF/heptane/ethylbenzene) (1.0 equiv.) and
N-alkyl isatoic anhydride (0.75 equiv.) in THF at −78 °C. The purity of the quinolones was determined by LC-MS (88–95%).
1-Methyl-2-(3′-decynyl)-4(1H)-quinolone (8a) was prepared from 3a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-methylisatoic anhydride (7a) (0.74 g, 4.2 mmol) in THF (10 mL) as a yellow oil (63%). IR (KBr, cm−1): 3406, 2929, 2857, 1627, 1600, 1500, 1469, 1177, 759. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.68 (t, J = 7.6 Hz, 1H, H-7), 7.52 (d, J = 8.0 Hz, 1H, H-8), 7.39 (t, J = 7.2 Hz, 1H, H-6), 6.32 (s, 1H, H-3), 3.78 (s, 3H, N–CH3), 2.95 (t, J = 7.2 Hz, 2H, H-1'), 2.57 (t, J = 6.8 Hz, 2H, H-2'), 2.12 (t, J = 6.8 Hz, 2H, H-5'), 1.44 (quint, J = 7.2 Hz, 2H, H-6'), 1.32–1.20 (m, 6H, H-7'-9'), 0.86 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 177.5 (C-4), 152.6 (C-2), 140.6 (C-8a), 132.2 (C-7), 126.8 (C-5), 126.6 (C-4a), 123.5 (C-6), 115.5 (C-8), 110.7 (C-3), 82.8 (C-4'), 77.2 (C-3'), 35.3 (N–CH3), 32.9 (C-1'), 31.6 (C-8'), 28.6 (C-7'), 28.4 (C-6'), 22.6 (C-9'), 18.7 (C-2'), 18.5 (C-5'), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 296 (100).
1-Ethyl-2-(3'-decynyl)-4(1H)-quinolone (8b) was prepared from 3a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-ethylisatoic anhydride (7b) (0.8 g, 4.2 mmol) in THF (10 mL) as a yellow oil (55%). IR (KBr, cm−1): 3428, 2929, 2854, 1624, 1600, 1490, 1468, 1430, 1308, 759. 1H-NMR δ: 8.46 (d, J = 8.0 Hz, 1H, H-5), 7.63 (t, J = 7.6 Hz, 1H, H-7), 7.55 (d, J = 8.0 Hz, 1H, H-8), 7.37 (t, J = 7.6 Hz, 1H, H-6), 6.34 (s, 1H, H-3), 4.33 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.96 (t, J = 7.2 Hz, 2H, H-1'), 2.61 (t, J = 6.8 Hz, 2H, H-2'), 2.13 (t, J = 6.8 Hz, 2H, H-5'), 1.45 (t, J = 7.2 Hz, 3H, N–CH2–CH3), 1.42 (quint, J = 6.8 Hz, 2H, H-6'), 1.30–1.21 (m, 6H, H-7'-9'), 0.86 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 177.4 (C-4), 152.6 (C-2), 140.5 (C-8a), 132.2 (C-7), 126.9 (C-5), 126.7 (C-4a), 123.4 (C-6), 115.4 (C-8), 110.9 (C-3), 82.7 (C-4'), 77.1 (C-3'), 41.2 (N–CH2–CH3), 33.0 (C-1'), 31.5 (C-8'), 28.7 (C-7'), 28.5 (C-6'), 22.5 (C-9'), 18.8 (C-2'), 18.7 (C-5'), 14.2 (N–CH2–CH3), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 310 (100).
1-(n-Propyl)-2-(3′-decynyl)-4(1H)-quinolone (8c) was prepared from 3a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.85 g, 4.2 mmol) in THF (10 mL) as a yellow oil (56%). IR (KBr, cm−1): 3420, 2929, 2856, 1627, 1600, 1489, 1468, 1428, 1177, 759. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.64 (t, J = 8.0 Hz, 1H, H-7), 7.46 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.29 (s, 1H, H-3), 4.13 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.91 (t, J = 7.6 Hz, 2H, H-1'), 2.58 (t, J = 7.6 Hz, 2H, H-2'), 2.12 (t, J = 7.6 Hz, 2H, H-5'), 1.84 (m, 2H, N–CH2–CH2–CH3), 1.44 (quint, J = 7.6 Hz, 2H, H-6'), 1.35–1.23 (m, 6H, H-7'-9'), 1.08 (t, J = 7.6 Hz, 3H, N–CH2–CH2–CH3), 0.85 (t, J = 7.2 Hz, 3H, H-10'). 13C-NMR δ: 175.4 (C-4), 152.7 (C-2), 140.7 (C-8a), 132.1 (C-7), 126.8 (C-5), 126.6 (C-4a), 123.3 (C-6), 115.5 (C-8), 110.9 (C-3), 82.7 (C-4'), 77.1 (C-3'), 47.8 (N–CH2–CH2–CH3), 33.2 (C-1'), 31.4 (C-8'), 28.7 (C-7'), 28.5 (C-6'), 22.5 (C-9'), 22.1 (N–CH2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.0 (C-10'), 11.0 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-Methyl-2-(3'-undecynyl)-4(1H)-quinolone (8d) was prepared from 3b (1.0 g, 5.2 mmol) in THF (15 mL), LDA (2.9 mL, 5.2 mmol) and N-methylisatoic anhydride (7a) (0.69 g, 3.9 mmol) in THF (10 mL) as a yellow semi solid (58%). IR (KBr, cm−1): 3420, 2928, 2855, 1628, 1600, 1500, 1469, 1177, 759. 1H-NMR δ: 8.43 (d, J = 8.0 Hz, 1H, H-5), 7.65 (t, J = 8.0 Hz, 1H, H-7), 7.49 (d, J = 8.4 Hz, 1H, H-8), 7.36 (t, J = 7.6 Hz, 1H, H-6), 6.27 (s, 1H, H-3), 3.76 (s, 3H, N–CH3), 2.92 (t, J = 7.2 Hz, 2H, H-1'), 2.55 (t, J = 6.8 Hz, 2H, H-2'), 2.11 (t, J = 6.8 Hz, 2H, H-5'), 1.44 (quint, J = 7.2 Hz, 2H, H-6'), 1.33–1.21 (m, 8H, H-7'-10'), 0.86 (t, J = 6.8 Hz, 3H, H-11'). 13C-NMR δ: 177.4 (C-4), 153.1 (C-2), 141.8 (C-8a), 132.2 (C-7), 126.6 (C-5), 126.4 (C-4a), 123.5 (C-6), 115.4 (C-8), 111.1 (C-3), 82.8 (C-4'), 76.9 (C-3'), 34.4 (N–CH3), 34.0 (C-1'), 31.7 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.7 (C-6'), 22.6 (C-10'), 18.6 (C-2'), 18.5 (C-5'), 14.1 (C-11'). ESI-MS m/z (rel. int.): [M+H]+ 310 (100).
1-Ethyl-2-(3'-undecynyl)-4(1H)-quinolone (8e) was prepared from 3b (1.0 g, 5.2 mmol) in THF (15 mL), LDA (2.9 mL, 5.2 mmol) and N-ethylisatoic anhydride (7b) (0.74 g, 3.9 mmol) in THF (10 mL) as a yellow semi solid (62%). IR (KBr, cm−1): 3434, 2930, 2851, 1621, 1600, 1490, 1468, 1431, 1309, 759. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.65 (t, J = 8.0 Hz, 1H, H-7), 7.51 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.2 Hz, 1H, H-6), 6.28 (s, 1H, H-3), 4.27 (q, J = 7.6 Hz, 2H, N–CH2–CH3), 2.91 (t, J = 7.2 Hz, 2H, H-1'), 2.59 (t, J = 6.8 Hz, 2H, H-2'), 2.12 (t, J = 6.8 Hz, 2H, H-5'), 1.44 (t, J = 7.2 Hz, 2H, N–CH2–CH3), 1.42 (quint, J = 6.8 Hz, 2H, H-6'), 1.31–1.21 (m, 8H, H-7'-10'), 0.85 (t, J = 6.8 Hz, 3H, H-11'). 13C-NMR δ: 177.1 (C-4), 151.9 (C-2), 141.1 (C-8a), 132.1 (C-7), 126.9 (C-5), 126.7 (C-4a), 123.2 (C-6), 115.4 (C-8), 110.8 (C-3), 82.4 (C-4'), 77.4 (C-3'), 41.3 (N–CH2–CH3), 33.0 (C-1'), 31.6 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.6 (C-6'), 22.6 (C-10'), 18.8 (C-2'), 18.7 (C-5'), 14.2 (N–CH2–CH3), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-(n-Propyl)-2-(3'-undecynyl)-4(1H)-quinolone (8f) was prepared from 3b (1.0 g, 5.2 mmol) in THF (15 mL), LDA (2.9 mL, 5.2 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.79 g, 3.9 mmol) in THF (10 mL) as a yellow oil (59%). IR (KBr, cm−1): 3426, 2929, 2856, 1627, 1600, 1488, 1468, 1427, 1177, 759. 1H-NMR δ: 8.46 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.6 Hz, 1H, H-7), 7.48 (d, J = 8.0 Hz, 1H, H-8), 7.37 (t, J = 7.6 Hz, 1H, H-6), 6.36 (s, 1H, H-3), 4.13 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.93 (t, J = 7.6 Hz, 2H, H-1'), 2.61 (t, J = 7.2 Hz, 2H, H-2'), 2.13 (t, J = 7.2 Hz, 2H, H-5'), 1.85 (sext, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 1.45 (quint, J = 7.2 Hz, 2H, H-6'), 1.33–1.21 (m, 8H, H-7'-10'), 1.08 (t, J = 7.2 Hz, 2H, N–CH2–CH2–CH3), 0.86 (t, J = 6.8 Hz, 3H, H-11'). 13C-NMR δ: 177.3 (C-4), 152.7 (C-2), 140.5 (C-8a), 132.3 (C-7), 126.7 (C-5), 126.5 (C-4a), 123.2 (C-6), 115.6 (C-8), 111.0 (C-3), 82.5 (C-4'), 77.0 (C-3'), 47.7 (N–CH2–CH2–CH3), 32.9 (C-1'), 31.5 (C-9'), 29.0 (C-8'), 28.7 (C-7'), 28.4 (C-6'), 22.6 (C-10'), 22.3 (N–CH2–CH2–CH3), 18.7 (C-2'), 18.6 (C-5'), 14.0 (C-11'), 11.1 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 338 (100).
1-Ethyl-2-(1'-decynyl)-4(1H)-quinolone (8g) was prepared from 6a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-ethylisatoic anhydride (7b) (0.80 g, 4.2 mmol) in THF (10 mL) as a light yellow semi solid (54%). IR (KBr, cm−1): 3372, 2928, 2855, 2234, 1625, 1598, 1488, 1421, 758. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.69 (t, J = 7.6 Hz, 1H, H-7), 7.50 (d, J = 8.0, Hz, 1H, H-8), 7.38 (t, J = 7.6 Hz, 1H, H-6), 6.58 (s, 1H, H-3), 4.53 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.53 (t, J = 7.2 Hz, 2H, H-3'), 1.67 (quint, J = 7.2 Hz, 2H, H-4'), 1.49 (t, J = 7.2 Hz, 2H, N–CH2–CH3), 1.33–1.22 (m, 10H, H-5'-9'), 0.89 (t, J = 7.2 Hz, 3H, H-10'). 13C-NMR δ: 177.1 (C-4), 141.3 (C-8a), 137.5 (C-2), 132.5 (C-7), 126.8 (C-4a), 126.6 (C-5), 123.5 (C-6), 115.5 (C-8), 115.1 (C-3), 111.3 (C-2'), 74.5 (C-1'), 42.4 (N–CH2–CH3), 31.8 (C-8'), 29.2 (C-7'), 29.1 (C-6'), 29.0 (C-5'), 28.1 (C-4'), 22.6 (C-9'), 19.3 (C-3'), 14.2 (N–CH2–CH3), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 310 (100).
1-(n-Propyl)-2-(1'-decynyl)-4(1H)-quinolone (8h) was prepared from 6a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.85 g, 4.2 mmol) in THF (10 mL) as a light yellow semi solid (51%). IR (KBr, cm−1): 3430, 2927, 2855, 2235, 1624, 1598, 1488, 1420, 1177, 758. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.2 Hz, 1H, H-7), 7.44 (d, J = 8.0 Hz, 1H, H-8), 7.36 (t, J = 7.2 Hz, 1H, H-6), 6.56 (s, 1H, H-3), 4.40 (t, J = 7.6 Hz, 2H, N–CH2–CH2–CH3), 2.51 (t, J = 6.8 Hz, 2H, H-3'), 1.86 (m, 2H, N–CH2–CH2–CH3), 1.65 (quint, J = 6.8 Hz, 2H, H-4'), 1.33–1.21 (m, 10H, H-5'-9'), 1.03 (t, J = 7.2 Hz, 3H, N–CH2–CH2–CH3), 0.89 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 177.1 (C-4), 141.0 (C-8a), 138.2 (C-2),132.5 (C-7), 126.7 (C-4a), 126.5 (C-5), 123.7 (C-6), 115.4 (C-8), 115.2 (C-3), 111.0 (C-2'), 75.1 (C-1'), 47.9 (N–CH2–CH2–CH3), 31.7 (C-8'), 29.1 (C-7'), 29.0 (C-6'), 29.0 (C-5'), 28.1 (C-4'), 22.6 (C-9'), 22.3 (N–CH2–CH2–CH3), 19.8 (C-3'), 14.0 (C-10'), 11.0 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-(n-Butyl)-2-(1'-decynyl)-4(1H)-quinolone (8i) was prepared from 6a (1.0 g, 5.6 mmol) in THF (15 mL), LDA (3.1 mL, 5.6 mmol) and N-(n-butyl)isatoic anhydride (7d) (0.91 g, 4.2 mmol) in THF (10 mL) as a light yellow oil (54%). IR (KBr, cm−1): 3428, 2928, 2856, 2233, 1622, 1597, 1490, 1466, 1422, 759. 1H-NMR δ: 8.43 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.6 Hz, 1H, H-7), 7.45 (d, J = 8.0, Hz, 1H, H-8), 7.33 (t, J = 7.6 Hz, 1H, H-6), 6.58 (s, 1H, H-3), 4.38 (t, J = 7.6 Hz, 2H, N–CH2–CH2–CH2–CH3), 2.53 (t, J = 6.8 Hz, 2H, H-3'), 1.78 (quint, J = 7.2 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.65 (quint, J = 6.8 Hz, 2H, H-4'), 1.48 (m, 2H, N–CH2–CH2–CH2–CH3), 1.31–1.24 (m, 10H, H-5'-9'), 1.04 (t, J = 7.6 Hz, 3H, N–CH2–CH2–CH2–CH3), 0.87 (t, J = 6.8 Hz, 3H, H-10’). 13C-NMR δ: 177.1 (C-4), 141.3 (C-8a), 137.3 (C-2),132.5 (C-7), 126.7 (C-4a), 126.5 (C-5), 123.7 (C-6), 115.7 (C-8), 115.0 (C-3), 111.0 (C-2'), 74.4 (C-1'), 48.4 (N–CH2–CH2–CH2–CH3), 31.8 (C-8'), 31.4 (N–CH2–CH2–CH2–CH3), 29.1 (C-7'), 29.0 (C-6'), 29.0 (C-5'), 28.1 (C-4'), 22.6 (C-9'), 20.9 (N–CH2–CH2–CH2–CH3), 19.3 (C-3'), 14.3 (N–CH2–CH2–CH2–CH3), 14.0 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 338 (100).
1-Methyl-2-(3'-dodecynyl)-4(1H)-quinolone (8j) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-methylisatoic anhydride (7a) (0.64 g, 3.6 mmol) in THF (10 mL) as a light yellow semi solid (60%). IR (KBr, cm−1): 3420, 2927, 2855, 1628, 1600, 1499, 1469, 759. 1H-NMR δ: 8.38 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.61 (t, J = 8.4 Hz, 1H, H-7), 7.45 (d, J = 8.4 Hz, 1H, H-8), 7.32 (t, J = 7.6 Hz, 1H, H-6), 6.19 (s, 1H, H-3), 3.71 (s, 3H, N–CH3), 2.87 (t, J = 7.6 Hz, 2H, H-1'), 2.52 (t, J = 7.6 Hz, 2H, H-2'), 2.07 (t, J = 7.6 Hz, 2H, H-5'), 1.43 (quint, J = 7.2 Hz, 2H, H-6'), 1.31–1.20 (m, 10H, H-7'-11'), 0.84 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.6 (C-4), 152.9 (C-2), 141.8 (C-8a), 132.1 (C-7), 126.5 (C-5), 126.3 (C-4a), 123.3 (C-6), 115.4 (C-8), 111.1 (C-3), 82.6 (C-4'), 77.0 (C-3'), 34.3 (N–CH3), 33.9 (C-1'), 31.8 (C-10'), 29.1 (C-9'), 29.1 (C-8'), 29.0 (C-7'), 28.8 (C-6'), 22.6 (C-11'), 18.6 (C-2'), 18.4 (C-5'), 14.0 (C-12'). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-Ethyl-2-(3'-dodecynyl)-4(1H)-quinolone (8k) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-ethylisatoic anhydride (7b) (0.69 g, 3.6 mmol) in THF (10 mL) as a white solid (55%). m.p. 66–68 °C. IR (KBr, cm−1): 3440, 2919, 2850, 1621, 1600, 1490, 1468, 1308, 769. 1H-NMR δ: 8.43 (d, J = 8.0 Hz, 1H, H-5), 7.64 (t, J = 8.0 Hz, 1H, H-7), 7.50 (d, J = 8.4 Hz, 1H, H-8), 7.34 (t, J = 7.2 Hz, 1H, H-6), 6.28 (s, 1H, H-3), 4.26 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.90 (t, J = 7.6 Hz, 2H, H-1'), 2.57 (t, J = 7.2 Hz, 2H, H-2'), 2.10 (t, J = 6.8 Hz, 2H, H-5'), 1.43 (t, J = 7.6 Hz, N–CH2–CH3), 1.41 (quint, J = 7.2 Hz, 2H, H-6'), 1.31–1.20 (m, 10H, H-7'-11'), 0.85 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.5 (C-4), 152.8 (C-2), 140.5 (C-8a), 132.1 (C-7), 126.9 (C-5), 126.7 (C-4a), 123.2 (C-6), 115.4 (C-8), 110.9 (C-3), 82.7 (C-4'), 77.0 (C-3'), 41.2 (N–CH2–CH3), 33.0 (C-1'), 31.7 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.8 (C-6'), 22.5 (C-11'), 18.8 (C-2'), 18.6 (C-5'), 14.1 (N–CH2–CH3), 14.0 (C-12'). ESI-MS m/z (rel. int.): [M+H]+ 338 (100).
1-(n-Propyl)-2-(3'-dodecynyl)-4(1H)-quinolone (8l) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.74 g, 3.6 mmol) in THF (10 mL) as a light yellow semi solid (51%). IR (KBr, cm−1): 3426, 2928, 2855, 1628, 1600, 1488, 1467, 1427, 759. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 8.0 Hz, 1H, H-7), 7.47 (d, J = 8.0 Hz, 1H, H-8), 7.36 (t, J = 7.6 Hz, 1H, H-6), 6.28 (s, 1H, H-3), 4.16 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.90 (t, J = 7.6 Hz, 2H, H-1'), 2.59 (t, J = 7.2 Hz, 2H, H-2'), 2.13 (t, J = 7.6 Hz, 2H, H-5'), 1.85 (sext, J = 7.2 Hz, 2H, N–CH2–CH2–CH3), 1.43 (quint, J = 7.6 Hz, 2H, H-6'), 1.33–1.21 (m, 10H, H-7'-11'), 1.07 (t, J = 7.2 Hz, 3H, N–CH2–CH2–CH3), 0.86 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 175.8 (C-4), 152.5 (C-2), 140.9 (C-8a), 132.2 (C-7), 126.7 (C-5), 126.5 (C-4a), 123.1 (C-6), 115.4 (C-8), 110.8 (C-3), 82.6 (C-4'), 77.1 (C-3'), 47.8 (N–CH2–CH2–CH3), 33.1 (C-1'), 31.6 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 29.0 (C-7'), 28.6 (C-6'), 22.6 (C-11'), 22.0 (N–CH2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.0 (C-14’), 10.9 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 352 (100).
1-(n-Butyl)-2-(3'-dodecynyl)-4(1H)-quinolone (8m) was prepared from 3c (1.0 g, 4.8 mmol) in THF (15 mL), LDA (2.7 mL, 4.8 mmol) and N-(n-butyl)isatoic anhydride (7d) (0.79 g, 3.6 mmol) in THF (10 mL) as a yellow oil (58%). IR (KBr, cm−1): 3425, 2927, 2855, 1628, 1600, 1488, 1467, 1427, 759. 1H-NMR δ: 8.44 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.65 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.48 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.27 (s, 1H, H-3), 4.13 (t, J =8.0 Hz, 2H, N–CH2–(CH2)2–CH3), 2.91 (t, J = 7.2 Hz, 2H, H-1'), 2.58 (t, J = 7.2 Hz, 2H, H-2'), 2.11 (m, 2H, H-5'), 1.80 (quint, J = 7.2 Hz, 2H, N–CH2–CH2–CH2–CH3), 1.46 (m, 2H, N-(CH2)2–CH2–CH3), 1.41 (quint, J = 7.2 Hz, 2H, H-6'), 1.32–1.23 (m, 10H, H-7'-11'), 1.03 (t, J = 7.2 Hz, 3H, N-(CH2)3–CH3), 0.85 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.4 (C-4), 152.8 (C-2), 140.7 (C-8a), 132.1 (C-7), 126.8 (C-5), 126.6 (C-4a), 123.3 (C-6), 115.6 (C-8), 110.8 (C-3), 82.7 (C-4'), 77.1 (C-3'), 47.6 (N–CH2–(CH2)2–CH3), 33.2 (C-1'), 31.8 (C-10'), 30.8 (N–CH2–CH2–CH2–CH3), 29.1 (C-9'), 29.0 (C-8'), 28.8 (C-7'), 28.8 (C-6'), 22.6 (C-11'), 20.1 (N–(CH2)2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.1 (C-14’), 13.8 (N–(CH2)3–CH3). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-Methyl-2-(1'-dodecynyl)-4(1H)-quinolone (8n) was prepared from 6b (1.5 g, 7.2 mmol) in THF (25 mL), LDA (4.0 mL, 7.2 mmol) and N-methylisatoic anhydride (7a) (0.96 g, 5.4 mmol) in THF (15 mL) as a light yellow semi-solid (51%). IR (KBr, cm−1): 3421, 2925, 2854, 2234, 1625, 1599, 1496, 1469, 757. 1H-NMR δ: 8.41 (d, J = 8.0 Hz, 1H, H-5), 7.66 (t, J = 7.6 Hz, 1H, H-7), 7.44 (d, J = 8.0, Hz, 1H, H-8), 7.36 (t, J = 7.6 Hz, 1H, H-6), 6.55 (s, 1H, H-3), 3.94 (s, 3H, N–CH3), 3.17 (t, J = 7.2 Hz, 2H, H-1'), 2.51 (t, J = 6.8 Hz, 2H, H-3'), 1.64 (quint, J = 6.8 Hz, 2H, H-4'), 1.46 (m, 2H, H-5'), 1.34–1.23 (m, 12H, H-6'-11'), 0.87 (t, J = 6.8 Hz, 3H, H-12'). 13C-NMR δ: 177.0 (C-4), 141.1 (C-8a), 137.4 (C-2), 132.4 (C-7), 126.8 (C-4a), 126.7 (C-5), 123.6 (C-6), 115.6 (C-8), 115.0 (C-3), 102.2 (C-2'), 74.6 (C-1'), 36.7 (N–CH3), 31.8 (C-10'), 29.5 (C-9'), 29.4 (C-8'), 29.2 (C-7'), 29.0 (C6'), 29.0 (C5'), 28.0 (C-4'), 22.6 (C-11'), 19.6 (C-3'), 14.0 (C-12'). ESI-MS m/z (rel. int.): [M+H]+ 324 (100).
1-Methyl-2-(3'-tetradecynyl)-4(1H)-quinolone (8o) was prepared from 3d (1.0 g, 4.2 mmol) in THF (15 mL), LDA (2.4 mL, 4.2 mmol) and N-methylisatoic anhydride (7a) (0.57 g, 3.2 mmol) in THF (10 mL) as a light yellow solid (60%). m.p. 43–45 °C. IR (KBr, cm−1): 3422, 2922, 2854, 1632, 1598, 1469, 1444, 760. 1H-NMR δ: 8.39 (d, J = 8.0 Hz, 1H, H-5), 7.62 (t, J = 8.0 Hz, 1H, H-7), 7.46 (d, J = 8.4 Hz, 1H, H-8), 7.33 (t, J = 7.2 Hz, 1H, H-6), 6.21 (s, 1H, H-3), 3.72 (s, 3H, N–CH3), 2.88 (t, J = 7.2 Hz, 2H, H-1'), 2.50 (t, J = 7.6 Hz, 2H, H-2’), 2.10 (t, J = 7.2 Hz, 2H, H-5'), 1.41 (quint, J = 6.8 Hz, 2H, H-6'), 1.34–1.21 (m, 14H, H-7'-13'), 0.85 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 177.6 (C-4), 152.9 (C-2), 141.8 (C-8a), 132.1 (C-7), 126.5 (C-5), 126.3 (C-4a), 123.3 (C-6), 115.4 (C-8), 111.1 (C-3), 82.7 (C-4'), 77.1 (C-3'), 34.3 (N–CH3), 33.9 (C-1'), 31.8 (C-12'), 29.2 (C-11'), 29.1 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 29.0 (C-7'), 28.8 (C-6'), 22.6 (C-13'), 18.6 (C-2'), 18.4 (C-5'), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 352 (100).
1-Ethyl-2-(3'-tetradecynyl)-4(1H)-quinolone (8p) was prepared from 3d (1.0 g, 4.2 mmol) in THF (15 mL), LDA (2.4 mL, 4.2 mmol) and N-ethylisatoic anhydride (7b) (0.61 g, 3.2 mmol) in THF (10 mL) as white needles (56%). m.p. 74–76 °C. IR (KBr, cm−1): 3424, 2917, 2850, 1621, 1600, 1468, 1430, 1308, 760. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.63 (t, J = 8.0 Hz, 1H, H-7), 7.50 (d, J = 8.4 Hz, 1H, H-8), 7.35 (t, J = 7.2 Hz, 1H, H-6), 6.29 (s, 1H, H-3), 4.24 (q, J = 7.2 Hz, 2H, N–CH2–CH3), 2.91 (t, J = 7.2 Hz, 2H, H-1'), 2.58 (t, J = 7.2 Hz, 2H, H-2'), 2.09 (t, J = 6.4 Hz, 2H, H-5'), 1.42 (t, J = 7.2 Hz, N–CH2–CH3), 1.40 (quint, J = 7.2 Hz, 2H, H-6'), 1.31–1.21 (m, 14H, H-7'-13'), 0.85 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 177.5 (C-4), 152.7 (C-2), 140.8 (C-8a), 132.1 (C-7), 126.6 (C-5), 126.4 (C-4a), 123.3 (C-6), 115.4 (C-8), 111.1 (C-3), 82.7 (C-4'), 77.0 (C-3'), 41.3 (N–CH2–CH3), 33.1 (C-1'), 31.8 (C-12'), 29.2 (C-11'), 29.2 (C-10'), 29.1 (C-9'), 29.0 (C-8'), 29.0 (C-7'), 28.8 (C-6'), 22.6 (C-13'), 18.8 (C-2'), 18.7 (C-5'), 14.2 (N–CH2–CH3), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-(n-Propyl)-2-(3'-tetradecynyl)-4(1H)-quinolone (8q) was prepared from 3d (1.0 g, 4.2 mmol) in THF (15 mL), LDA (2.4 mL, 4.2 mmol) and N-(n-propyl)isatoic anhydride (7c) (0.66 g, 3.2 mmol) in THF (10 mL) as a light yellow semi-solid (55%). IR (KBr, cm−1): 3430, 2926, 2854, 1629, 1600, 1488, 1467, 1227, 759. 1H-NMR δ: 8.48 (d, J = 8.0 Hz, 1H, H-5), 7.67 (t, J = 7.6 Hz, 1H, H-7), 7.49 (d, J = 8.4 Hz, 1H, H-8), 7.38 (t, J = 7.6 Hz, 1H, H-6), 6.43 (s, 1H, H-3), 4.17 (t, J = 8.0 Hz, 2H, N–CH2–CH2–CH3), 2.94 (t, J = 7.6 Hz, 2H, H-1'), 2.59 (t, J = 7.2 Hz, 2H, H-2'), 2.11 (t, J = 7.2 Hz, 2H, H-5'), 1.86 (sext, J = 7.6 Hz, 2H, N–CH2–CH2–CH3), 1.43 (quint, J = 7.2 Hz, 2H, H-6'), 1.33–1.22 (m, 14H, H-7'-13'), 1.09 (t, J = 7.2 Hz, 2H, N–CH2–CH2–CH3), 0.88 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 175.4 (C-4), 152.5 (C-2), 141.0 (C-8a), 132.1 (C-7), 126.7 (C-5), 126.5 (C-4a), 123.3 (C-6), 115.3 (C-8), 111.0 (C-3), 82.6 (C-4'), 77.0 (C-3’), 47.6 (N–CH2–CH2–CH3), 33.1 (C-1'), 31.5 (C-12'), 29.3 (C-11'), 29.2 (C-10'), 29.2 (C-9'), 29.1 (C-8'), 29.0 (C-7'), 28.4 (C-6'), 22.6 (C-13'), 22.1 (N–CH2–CH2–CH3), 18.8 (C-2'), 18.6 (C-5'), 14.0 (C-14'), 11.0 (N–CH2–CH2–CH3). ESI-MS m/z (rel. int.): [M+H]+ 380 (100).
1-Methyl-2-(1′-tetradecynyl)-4(1H)-quinolone (8r) was prepared from 6c (1.5 g, 6.4 mmol) in THF (25 mL), LDA (3.5 mL, 6.4 mmol) and N-methylisatoic anhydride (7a) (0.85 g, 4.8 mmol) in THF (15 mL) as a white solid (57%). M.p. 41–43 °C. IR (KBr, cm−1): 3405, 2923, 2849, 2233, 1622, 1595, 1496, 1468, 777. 1H-NMR δ: 8.38 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.63 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.42 (d, J = 8.0 Hz, 1H, H-8), 7.33 (t, J = 7.6 Hz, 1H, H-6), 6.51 (s, 1H, H-3), 3.92 (s, 3H, N–CH3), 2.49 (t, J = 7.2 Hz, 2H, H-3'), 1.63 (quint, J = 7.2 Hz, 2H, H-4'), 1.44 (m, 2H, H-5'), 1.34–1.21 (m, 16H, H-6'-13'), 0.85 (t, J = 6.8 Hz, 3H, H-14'). 13C-NMR δ: 177.1 (C-4), 141.1 (C-8a), 137.3 (C-2), 132.3 (C-7), 126.8 (C-4a), 126.6 (C-5), 123.6 (C-6), 115.6 (C-8), 115.0 (C-3), 102.0 (C-2'), 74.8 (C-1'), 36.7 (N–CH3), 31.8 (C-12'), 29.6 (C-11'), 29.6 (C-10'), 29.5 (C-9'), 29.4 (C-8'), 29.2 (C-7'), 29.0 (C6'), 28.9 (C5'), 27.9 (C-4'), 22.6 (C-13'), 19.6 (C-3'), 14.0 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 352 (100).
1-Methyl-2-(3′-hexadecynyl)-4(1H)-quinolone (8s) was prepared from 3e (1.5 g, 5.7 mmol) in THF (25 mL), LDA (3.2 mL, 5.7 mmol) and N-methylisatoic anhydride (7a) (0.76 g, 4.3 mmol) in THF (15 mL) as a light yellow solid (59%). M.p. 58–61 °C. IR (KBr, cm−1): 3432, 2922, 2853, 1631, 1597, 1469, 1444, 761. 1H-NMR δ: 8.46 (d, J = 8.0 Hz, 1H, H-5), 7.73 (t, J = 7.6 Hz, 1H, H-7), 7.58 (d, J = 8.0 Hz, 1H, H-8), 7.44 (t, J = 7.6 Hz, 1H, H-6), 6.56 (s, 1H, H-3), 3.85 (s, 3H, N–CH3), 3.00 (t, J = 7.6 Hz, 2H, H-1'), 2.60 (t, J = 7.6 Hz, 2H, H-2’), 2.12 (t, J = 7.2 Hz, 2H, H-5'), 1.41 (m, 2H, H-6'), 1.32–1.19 (m, 18H, H-7'-15'), 0.88 (t, J = 6.4 Hz, 3H, H-16'). 13C-NMR δ: 175.6 (C-4), 155.3 (C-2), 141.7 (C-8a), 133.3 (C-7), 126.4 (C-5), 126.2 (C-4a), 124.4 (C-6), 115.9 (C-8), 110.5 (C-3), 83.4 (C-4'), 76.5 (C-3'), 35.6 (N–CH3), 34.1 (C-1'), 31.9 (C-14'), 29.7 (C-13'), 29.6 (C-12'), 29.6 (C-11'), 29.5 (C-10'), 29.3 (C-9'), 29.1 (C-8'), 28.9 (C-7'), 28.9 (C-6'), 22.7 (C-15'), 18.8 (C-2'), 18.6 (C-5'), 14.1 (C-16'). ESI-MS m/z (rel. int.): [M+H]+ 380 (100).
1-Methyl-2-(1'-pentadecynyl)-4(1H)-quinolone (8t) was prepared from 6d (1.5 g, 6.0 mmol) in THF (25 mL), LDA (3.3 mL, 6.0 mmol) and N-methylisatoic anhydride (7a) (0.80 g, 4.5 mmol) in THF (15 mL) as white needles (48%). M.p. 67–69 °C. IR (KBr, cm−1): 3401, 2915, 2851, 2239, 1618, 1596, 1472, 748. 1H-NMR δ: 8.42 (d, J = 8.4, Hz, 1H, H-5), 7.69 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.48 (d, J = 8.0 Hz, 1H, H-8), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.55 (s, 1H, H-3), 3.96 (s, 3H, N–CH3), 2.52 (t, J = 7.2 Hz, 2H, H-3'), 1.66 (quint, J = 7.6 Hz, 2H, H-4'), 1.46 (quint, J = 7.2 Hz, 2H, H-5'), 1.35–1.21 (m, 18H, H-6'-14'), 0.87 (t, J = 6.8 Hz, 3H, H-15'). 13C-NMR δ: 177.2 (C-4), 141.0 (C-8a), 136.6 (C-2), 132.2 (C-7), 126.7 (C-4a), 126.6 (C-5), 123.7 (C-6), 115.7 (C-8), 115.1 (C-3), 102.1 (C-2'), 74.9 (C-1'), 36.5 (N–CH3), 31.8 (C-13'), 29.6 (C-12'), 29.5 (C-11'), 29.5 (C-10'), 29.3 (C-9'), 29.3 (C-8'), 29.1 (C-7'), 29.1 (C6'), 28.9 (C5'), 28.0 (C-4'), 22.6 (C-14'), 19.5 (C-3'), 14.1 (C-14'). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-Cyclopropyl-2-[(E)-12-bromodec-1'-enyl]-4(1H)-quinolone (18a) was prepared from 17a (1.0 g, 3.5 mmol) in THF (15 mL), LDA (1.9 mL, 3.5 mmol) and N-cyclopropylisatoic anhydride (12) (0.52 g, 2.6 mmol) in THF (10 mL) as a light yellow solid (49%). M.p. 85–87 °C. IR (KBr, cm−1): 3425, 2926, 2850, 1648, 1616, 1594, 1475, 1416, 1310, 1137, 1034, 966, 888, 760. 1H-NMR δ: 8.39 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.90 (d, J = 8.4 Hz, 1H, H-8), 7.64 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.67 (d, J = 16.0 Hz, 1H, H-1'), 6.55 (s, 1H, H-3), 6.45 (dt, J = 16.0, 6.8 Hz, 1H, H-2'), 3.41 (t, J = 6.8 Hz, 2H, H-12'), 3.28 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.30 (q, J = 6.8 Hz, 2H, H-3'), 1.83 (quint, J = 6.8 Hz, 2H, H-11'), 1.51 (quint, J = 6.8 Hz, 2H, H-4'), 1.41–1.23 (m, 12H, H-5'-10'), 0.90 (m, 4H, N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 153.0 (C-2), 142.2 (C-2'), 139.6 (C-8a), 131.5 (C-7), 126.4 (C-4a), 126.2 (C-5), 124.9 (C-1'), 123.4 (C-6), 117.4 (C-8), 108.2 (C-3), 34.1 (C-12'), 33.1 (C-3'), 32.7 (C-11'), 29.9 (C-8'), 29.4 (C-7'), 29.4 (C-9'), 29.3 (C-6'), 29.1 (C-5'), 28.6 (C-4'), 28.1 (C-10'), 24.7 (N–CH–(CH2)2), 2×12.4 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H+2]+ 432 (100), [M+H]+ 430 (94).
1-Cyclopropyl-2-[(E)-13-bromotridec-1'-enyl)-4(1H)-quinolone (18b) was prepared from 17b (1.0 g, 3.3 mmol) in THF (15 mL), LDA (1.8 mL, 3.3 mmol) and N-cyclopropylisatoic anhydride (12) (0.5 g, 2.5 mmol) in THF (10 mL) as a light yellow solid (55%). M.p. 61–63 °C. IR (KBr, cm−1): 3422, 3020, 2918, 2851, 1653, 1630, 1596, 1571, 1479, 1463, 1413, 1135, 1034, 973, 760. 1H-NMR δ: 8.38 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.89 (d, J = 8.4 Hz, 1H, H-8), 7.64 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.35 (t, J = 7.6 Hz, 1H, H-6), 6.66 (d, J = 16.0 Hz, 1H, H-1'), 6.50 (s, 1H, H-3), 6.44 (dt, J = 16.0, 6.8 Hz, 1H, H-2'), 3.41 (t, J = 6.8 Hz, 2H, H-13'), 3.27 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.30 (q, J = 6.8 Hz, 2H, H-3'), 1.85 (quint, J = 6.8 Hz, 2H, H-12'), 1.51 (quint, J = 6.8 Hz, 2H, H-4'), 1.43–1.23 (m, 14H, H-5'-11'), 0.90 (m, 4H, N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 152.9 (C-2), 142.1 (C-2'), 139.5 (C-8a), 131.4 (C-7), 126.4 (C-4a), 126.2 (C-5), 124.9 (C-1'), 123.3 (C-6), 117.4 (C-8), 108.2 (C-3), 34.1 (C-13'), 33.1 (C-3'), 32.7 (C-12'), 29.5 (C-9'), 29.5 (C-8'), 29.3 (C-10'), 29.3 (C-7'), 29.2 (C-6'), 28.7 (C-5'), 28.6 (C-4'), 28.1 (C-11'), 24.9 (N–CH–(CH2)2), 2 × 12.4 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H+2]+ 446 (100), [M+H]+ 444 (94).
1-Methyl-2-[(E)-12-bromodec-1'-enyl]-4(1H)-quinolone (19a) was prepared from 17a (1.5 g, 5.2 mmol) in THF (25 mL), LDA (2.9 mL, 5.2 mmol) and N-methylisatoic anhydride (7a) (0.69 g, 3.9 mmol) in THF (10 mL) as a yellow semi solid (63%). IR (KBr, cm−1): 3422, 2925, 2852, 1620, 1597, 1467, 1438, 761. 1H-NMR δ: 8.44 (d, J = 8.0 Hz, 1H, H-5), 7.67 (t, J = 7.2 Hz, 1H, H-7), 7.49 (t, J = 8.4 Hz, 1H, H-8), 7.38 (t, J = 7.2 Hz, 1H, H-6), 6.46 (s, 1H, H-3), 6.43 (d, J = 16.0 Hz, 1H, H-1'), 6.37 (dt, J = 16.0, 6.4 Hz, 1H, H-2'), 3.76 (s, 3H, N–CH3), 3.41 (t, J = 6.4 Hz, 2H, H-12'), 2.28 (q, J = 6.8 Hz, 2H, H-3'), 1.85 (quint, J = 6.8 Hz, 2H, H-11'), 1.50 (quint, J = 6.8 Hz, 2H, H-4'), 1.41 (quint, J = 6.8 Hz, 2H, H-10'), 1.37–1.23 (m, 10H, H-5'-9'). 13C-NMR δ: 177.9 (C-4), 152.5 (C-2), 142.0 (C-2'), 141.4 (C-8a), 132.2 (C-7), 126.6 (C-4a), 126.5 (C-5), 123.8 (C-1'), 123.5 (C-6), 115.5 (C-8), 109.4 (C-3), 35.5 (N–CH3), 34.1 (C-12'), 33.1 (C-3'), 32.7 (C-11'), 29.4 (C-8'), 29.3 (C-9'), 29.3 (C-7'), 29.1 (C-6'), 28.7 (C-5'), 28.5 (C-4'), 28.1 (C-10'). ESI-MS m/z (rel. int.): [M+H]+ 404 (100), [M+H+2]+ 406 (92).
1-Methyl-2-[(E)-13-bromotridec-1'-enyl)-4(1H)-quinolone (19b) was prepared from 17b (1.0 g, 3.3 mmol) in THF (15 mL), LDA (1.8 mL, 3.3 mmol) and N-methylisatoic anhydride (7a) (0.44 g, 2.5 mmol) in THF (10 mL) as a yellow semi solid (47%). IR (KBr, cm−1): 3420, 2925, 2852, 1622, 1597, 1496, 1468, 760. 1H-NMR δ: 8.45 (d, J = 8.0 Hz, 1H, H-5), 7.70 (t, J = 7.6 Hz, 1H, H-7), 7.52 (t, J = 8.0 Hz, 1H, H-8), 7.40 (t, J = 7.6 Hz, 1H, H-6), 6.48 (s, 1H, H-3), 6.44 (d, J = 16.0 Hz, 1H, H-1'), 6.37 (dt, J = 16.0, 6.4 Hz, 1H, H-2'), 3.79 (s, 3H, N–CH3), 3.41 (t, J = 6.8 Hz, 2H, H-13'), 2.29 (q, J = 7.2 Hz, 2H, H-3'), 1.86 (quint, J = 6.8 Hz, 2H, H-12'), 1.52 (quint, J = 6.8 Hz, 2H, H-4'), 1.43 (quint, J = 6.8 Hz, 2H, H-11'), 1.37–1.22 (m, 12H, H-5'-10'). 13C-NMR δ: 177.4 (C-4), 152.3 (C-2), 142.4 (C-2'), 141.4 (C-8a), 132.4 (C-7), 126.7 (C-4a), 126.6 (C-5), 123.9 (C-1'), 123.6 (C-6), 115.5 (C-8), 109.4 (C-3), 35.6 (N–CH3), 34.1 (C-13'), 33.2 (C-3'), 32.8 (C-12'), 29.5 (C-9'), 29.5 (C-8'), 29.3 (C-10'), 29.3 (C-7'), 29.1 (C-6'), 28.7 (C-5'), 28.6 (C-4'), 28.1 (C-11'). ESI-MS m/z (rel. int.): [M+H]+ 418 (100), [M+H+2]+ 420 (94).
1-Cyclopropyl-2-[(E)-1′-tridecenyl]-4(1H)-quinolone (22a) was prepared from 21a (1.5 g, 6.7 mmol) in THF (25 mL), LDA (3.7 mL, 6.7 mmol) and N-cyclopropylisatoic anhydride (12) (1.0 g, 5.0 mmol) in THF (20 mL) as white needles (56%). M.p. 86–88 °C. IR (KBr, cm−1): 3425, 2922, 2851, 1617, 1595, 1571, 1479, 1465, 1420, 1310, 1136, 1031, 966, 759. 1H-NMR δ: 8.37 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.88 (d, J = 8.4 Hz, 1H, H-8), 7.62 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.33 (t, J = 8.0 Hz, 1H, H-6), 6.65 (d, J = 16.0 Hz, 1H, H-1'), 6.48 (s, 1H, H-3), 6.42 (dt, J = 16.0, 6.8 Hz, 1H, H-2′), 3.26 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.29 (q, J = 6.8 Hz, 2H, H-3′), 1.52 (quint, J = 6.8 Hz, 2H, H-4'), 1.36–1.24 (m, 16H, H-5'-12'), 0.86–0.90 (m, 7H, H-13', N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 152.8 (C-2), 142.1 (C-8a), 139.3 (C-2'), 131.4 (C-7), 126.5 (C-4a), 126.1 (C-5), 124.9 (C-1'), 123.3 (C-6), 117.4 (C-8), 108.2 (C-3), 33.1 (C-3'), 31.8 (C-11'), 29.8 (N–CH–(CH2)2), 29.6 (C-10'), 29.5 (C-9), 29.5 (C-8'), 29.4 (C-7'), 29.3 (C6'), 29.2 (C5'), 28.6 (C-4'), 22.6 (C-12'), 14.0 (C-13'), 12.3 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H]+ 366 (100).
1-Cyclopropyl-2-[(E)-1′-tetradecenyl]-4(1H)-quinolone (22b) was prepared from 21b (1.5 g, 6.3 mmol) in THF (25 mL), LDA (3.5 mL, 6.3 mmol) and N-cyclopropylisatoic anhydride (12) (0.96 g, 4.7 mmol) in THF (20 mL) as white crystals (50%). M.p. 96–98 °C. IR (KBr, cm−1): 3422, 2919, 2848, 1620, 1598, 1572, 1482, 1465, 1419, 1306, 1132, 1037, 966, 750. 1H-NMR δ: 8.36 (d, J = 8.0 Hz, 1H, H-5), 7.87 (d, J = 8.4 Hz, 1H, H-8), 7.62 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.32 (t, J = 8.0 Hz, 1H, H-6), 6.65 (d, J = 16.0 Hz, 1H, H-1'), 6.46 (s, 1H, H-3), 6.42 (dt, J = 16.0, 6.8 Hz, 1H, H-2'), 3.25 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 2.28 (q, J = 6.8 Hz, 2H, H-3'), 1.51 (quint, J = 6.8 Hz, 2H, H-4'), 1.37–1.21 (m, 18H, H-5'-13'), 0.86–0.91 (m, 7H, H-14', N–CH–(CH2)2). 13C-NMR δ: 178.2 (C-4), 152.8 (C-2), 142.1 (C-8a), 139.3 (C-2'), 131.3 (C-7), 126.5 (C-4a), 126.1 (C-5), 124.9 (C-1'), 123.2 (C-6), 117.4 (C-8), 108.3 (C-3), 33.1 (C-3'), 31.8 (C-12'), 29.8 (N–CH–(CH2)2), 29.6 (C-11'), 29.6 (C-10'), 29.6 (C-9), 29.5 (C-8'), 29.4 (C-7'), 29.3 (C6'), 29.2 (C5'), 28.7 (C-4'), 22.6 (C-13'), 14.1 (C-14'), 12.3 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H]+ 380 (100).
1-Cyclopropyl-2-(3′-undecynyl)-4(1H)-quinolone (23) was prepared from 3b (1.5 g, 7.7 mmol) in THF (25 mL), LDA (4.3 mL, 7.7 mmol) and N-cyclopropylisatoic anhydride (12) (1.17 g, 5.8 mmol) in THF (25 mL) as a yellow oil (48%). IR (KBr, cm−1): 3424, 2928, 2855, 1628, 1601, 1553, 1481, 1466, 1420, 1311, 1132, 1044, 759. 1H-NMR δ: 8.34 (dd, J = 8.0, 1.6 Hz, 1H, H-5), 7.87 (d, J = 8.4 Hz, 1H, H-8), 7.60 (td, J = 8.0, 1.6 Hz, 1H, H-7), 7.31 (t, J = 6.8 Hz, 1H, H-6), 6.25 (s, 1H, H-3), 3.27 (sept, J = 4.0 Hz, 1H, N–CH–(CH2)2), 3.17 (t, J = 7.2 Hz, 2H, H-1'), 2.54 (m, 2H, H-2'), 2.07 (m, 2H, H-5'), 1.41 (quint, J = 6.8 Hz, 2H, H-6'), 1.26–1.15 (m, 6H, H-7'-9'), 0.93 (m, 4H, N–CH–(CH2)2), 0.85 (t, J = 6.8 Hz, 3H, H-10'). 13C-NMR δ: 178.1 (C-4), 155.6 (C-2), 142.8 (C-8a), 131.1 (C-7), 126.3 (C-4a), 126.1 (C-5), 123.1 (C-6), 117.6 (C-8), 111.0 (C-3), 82.9 (C-4'), 77.5 (C-3'), 33.0 (C-1'), 31.6 (C-8'), 29.1 (C-7'), 28.7 (C-6'), 28.7 (C-5'), 26.8 (N–CH–(CH2)2), 22.5 (C-9'), 18.6 (C-2'), 14.0 (C-10'), 12.3 (N–CH–(CH2)2). ESI-MS m/z (rel. int.): [M+H]+ 336 (100).