The Genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and Pharmacological Characteristics
Abstract
:1. Introduction
2. Phytochemicals and Pharmacological Aspects
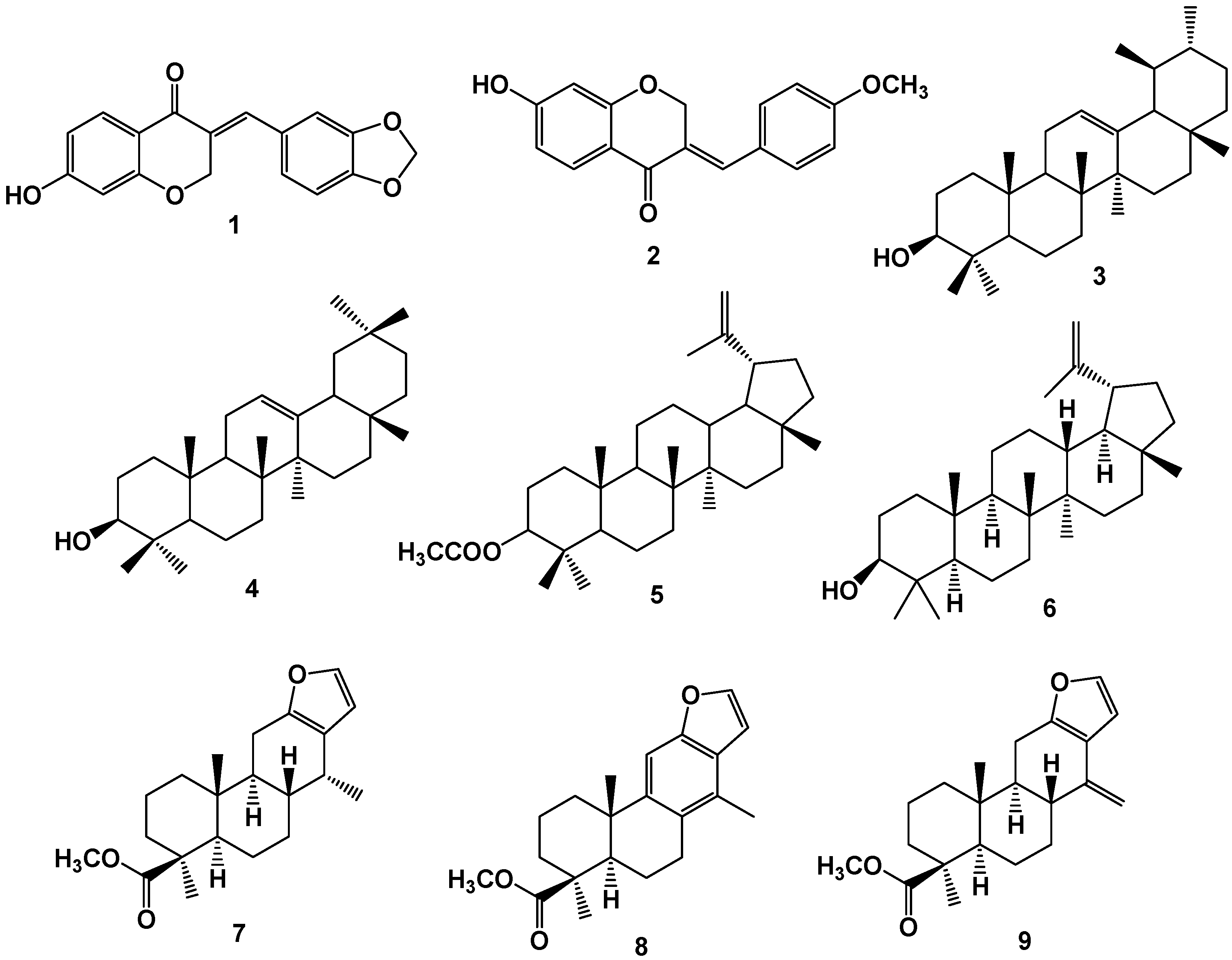
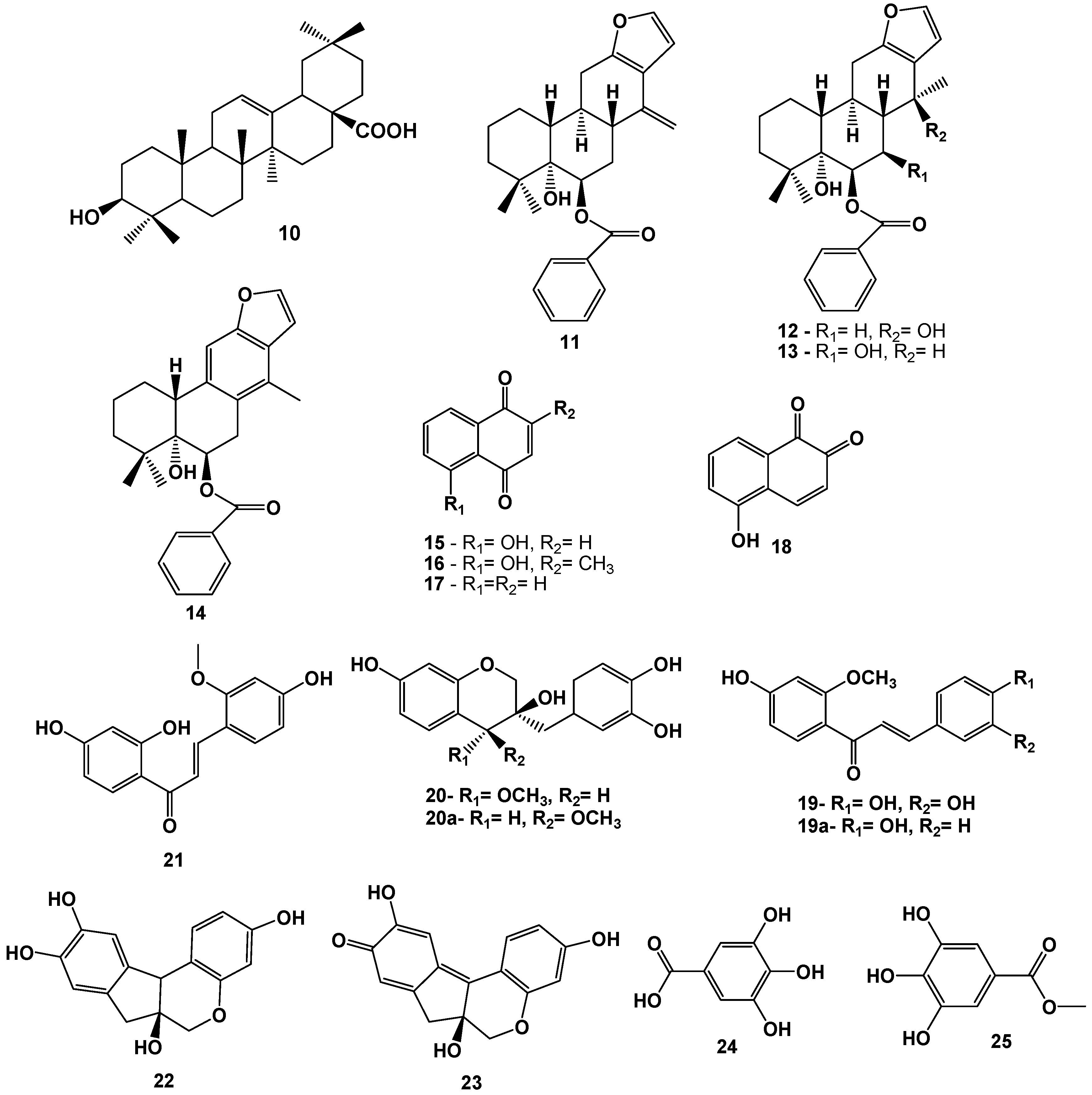
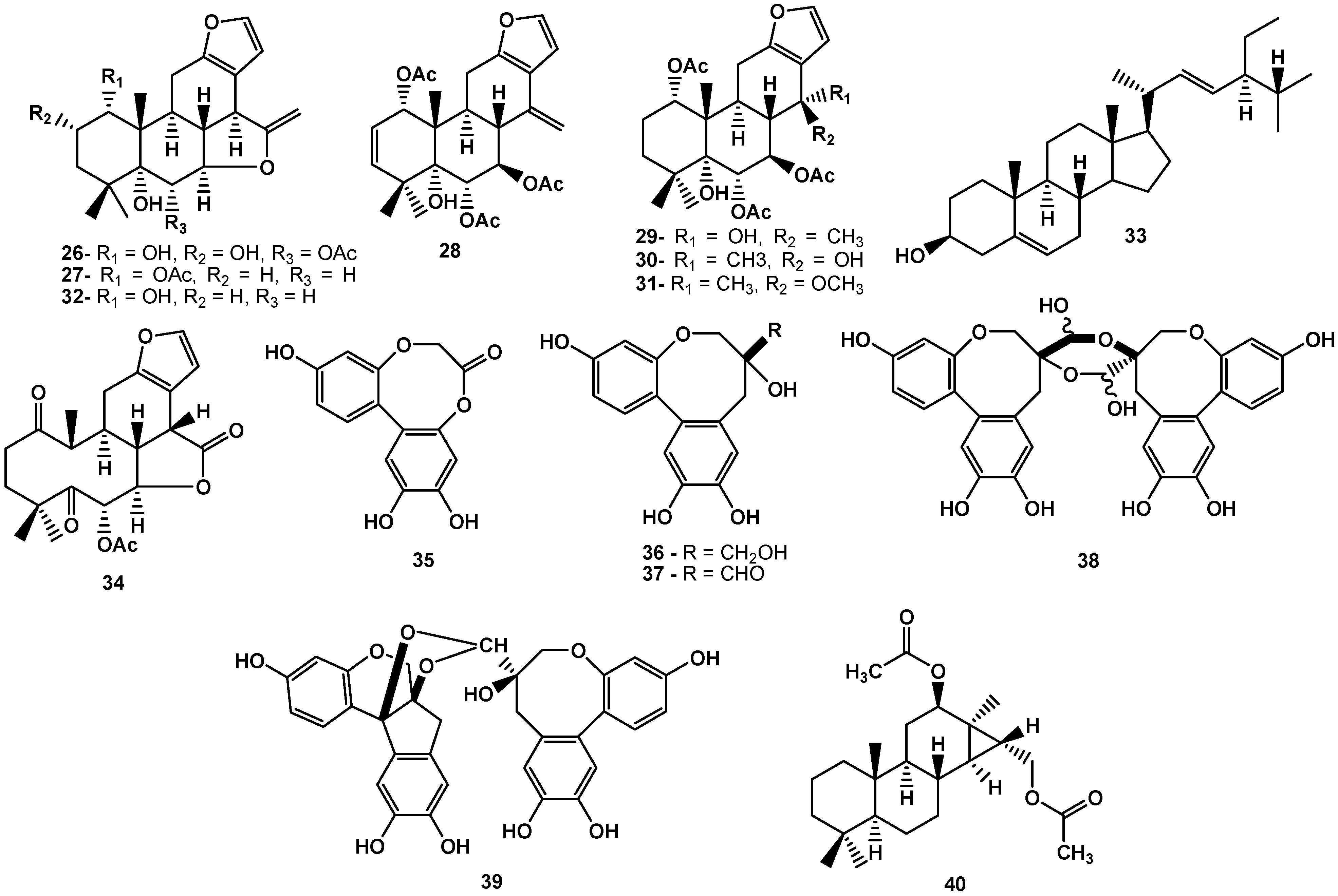
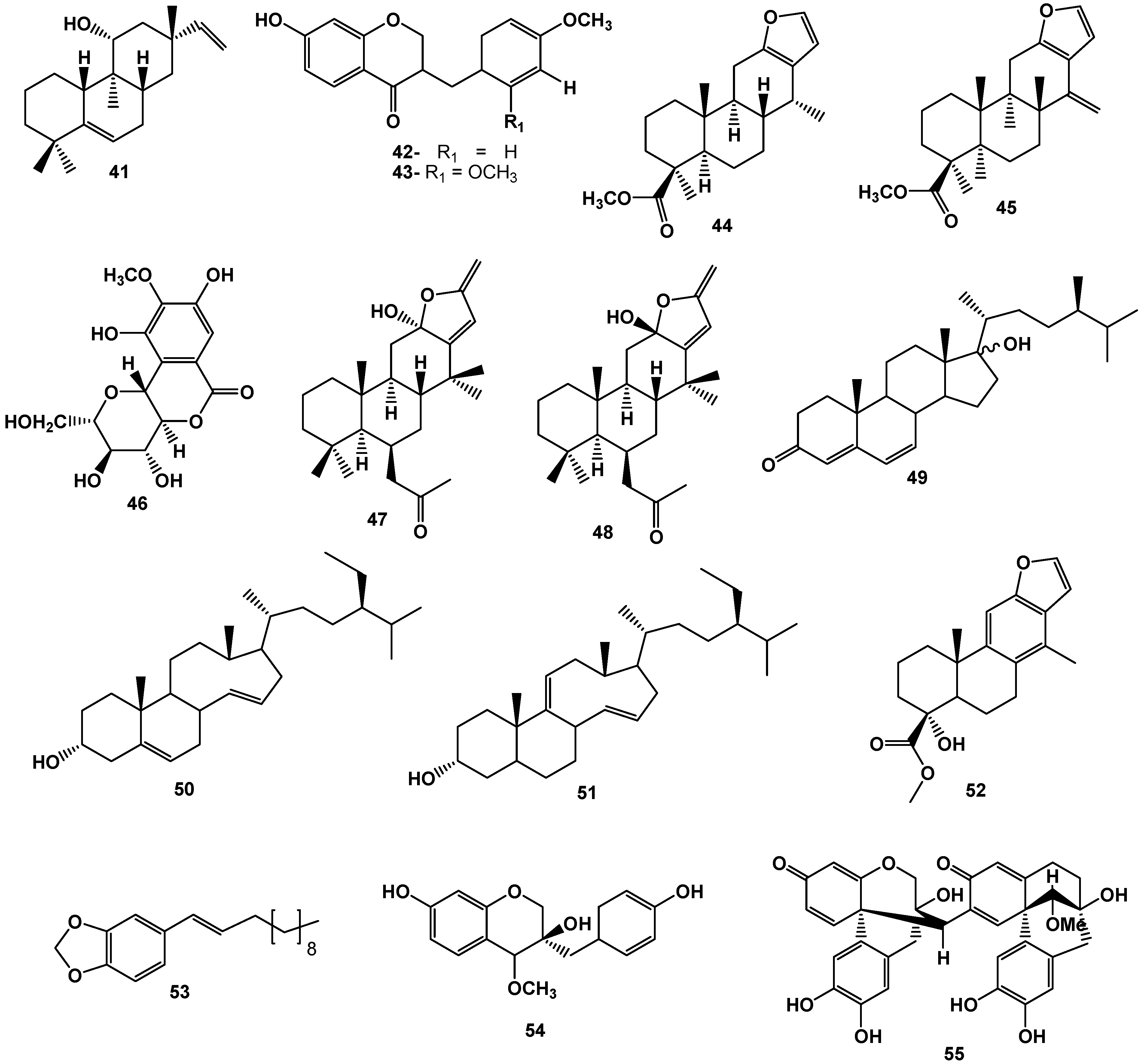
3. Conclusions
Acknowledgments
References and Notes
- Rates, S.M.K. Plants as source of drugs. Toxicon 2000, 39, 603–613. [Google Scholar] [CrossRef]
- Pinto, A.C.; Silva, D.H.S.; Bolzani, V.S.; Lopes, N.P.; Epifanio, R.A. Produtos naturais: Atividade, desafios e perspectivas. Quim. Nova 2002, 25, 45–61. [Google Scholar] [CrossRef]
- Niero, R.; Malheiros, A.; Bittencourt, C.M.S.; Biavatti, M.W.; Leite, S.N.; Cechinel-Filho, V. Aspectos químicos e biológicos de plantas medicinais e considerações sobre fitoterápicos. In Ciências Farmacêuticas: Contribuição ao desenvolvimento de novos fármacos e medicamentos; Bresolin, T.M.B., Cechinel Filho, V., Eds.; Univali: Itajaí, Brazil, 2003; pp. 10–56. [Google Scholar]
- Elisabetsky, E. Sociopolitical, economical and ethical issues in medicinal plant research. J. Ethnopharmacol. 1991, 32, 235–239. [Google Scholar] [CrossRef]
- Pinto, A.C. O Brasil dos viajantes e exploradores e a Química de Produtos Naturais Brasileira. Quim Nova 1995, 18, 608–615. [Google Scholar]
- Srinivas, K.V.N.S.; Rao, Y.K.; Das, I.M.B.; Krishna, K.V.S.R.; Kishore, K.H.; Murty, U.S.N. Flavanoids from Caesalpinia pulcherrima. Phytochemistry 2003, 63, 789–793. [Google Scholar]
- Nagumo, S.; Whasiyama, M.; Sasaki, Y.; Hosokawa, T. Anti-inflammatory constituents of Sappan lignum. Biol. Pharm. Bull. 2009, 32, 941–944. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Ata, A.; Samarasekera, R. Glutathione S-transferase inhibiting chemical constituents of Caesalpinia bonduc. Chem. Pharm. Bull. 2007, 55, 442–445. [Google Scholar] [CrossRef]
- Hemalatha, K.; Kiran, A.S.; Bannappa, U.; Satyanarayana, D. Analgesic activity of Caesalpinia sappan heartwood. Pharm. Biol. 2007, 45, 360–362. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; Teixeira, J.R.M.; Souza, P.J.C.; Bastos, J.K.; Filho, D.S.; Sarti, S.J. Preliminary studies of analgesic and anti-inflammatory properties of Caesalpinia ferrea crude extract. J. Ethnopharmacol. 1996, 53, 175–178. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A.; Mehta, P.; Vyas, S.P.; Shukla, S.; Bajpai, V.K. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food Chem. Toxicol. 2010, 48, 61–64. [Google Scholar] [CrossRef]
- Archana, P.; Tandan, S.K.; Chandra, S.; Lal, J. Antipyretic and analgesic activities of Caesalpinia bonducella seed kernel extract. Phytother. Res. 2005, 19, 376–381. [Google Scholar] [CrossRef]
- Devi, R.A.; Tandan, S.K.; Kumar, D.; Dudhgaonkar, S.P.; Lal, J. Analgesic activity of Caesalpinia bonducella flower extract. Pharm. Biol. 2008, 46, 668–672. [Google Scholar]
- Jabbar, A.; Zaman, M.A.; Iqbal, Z.; Yassen, M.; Shamin, A. Antihelmintic activity of Chenopodium album (L.) and Caesalpinia crista (L.) against trichostrongylid nematodes of sheep. J. Ethnopharmacol. 2007, 114, 86–91. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A.; John, J.; Singh, S.; Mehta, P.; Vyas, S.P. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem. Toxicol. 2009, 47, 1848–1851. [Google Scholar] [CrossRef]
- Roengsumran, S.; Limsuwankesorn, S.; Ngamrojnavanich, N.; Petson, A.; Chaichantipyuth, C.; Ishikawa, T. Cassane diterpenoid from Caesalpinia major. Phytochemistry 2000, 53, 841–844. [Google Scholar] [CrossRef]
- Das, B.; Trirupathi, P.; Ravikanth, B.; Kumar, R.A.; Sarma, A.L.S.; Basha, S.J. Isolation, synthesis, and bioactivity of homoisoflavonoids from Caesalpinia pulcherrima. Chem. Pharm. Bull. 2009, 57, 1139–1141. [Google Scholar] [CrossRef]
- Sudhakar, M.; Rao, C.V.; Rao, P.M.; Raju, D.B.; Venkateswarlu, Y. Antimicrobial activity of Caesalpinia pulcherrima, Euphorbia hirtaand Asystasia gangeticum. Fitoterapia 2006, 77, 378–380. [Google Scholar] [CrossRef]
- Saeed, M.A.; Sabir, A.W. Antibacterial activity of Caesalpinia bonducella seeds. Fitoterapia 2001, 72, 807–809. [Google Scholar] [CrossRef]
- Dickson, R.A.; Houghton, P.J.; Hylands, P.J. Antibacterial and antioxidant cassane diterpenoids from Caesalpinia benthamiana. Phytochemistry 2007, 68, 1436–1441. [Google Scholar] [CrossRef]
- Woldemichael, G.M.; Singh, M.P.; Maiese, W.M.; Timmermann, B.N. Constituents of antibacterial extract of Caesalpinia paraguaiensis Burk. Z. Naturforsch 2003, 58c, 70–75. [Google Scholar]
- Ragasa, C.Y.; Hofileña, J.G.; Rideout, J.A. New furanoid diterpenes from Caesalpinia pulcherrima. J. Nat. Prod. 2002, 65, 1107–1110. [Google Scholar] [CrossRef]
- Sgariglia, M.A.; Soberón, J.R.; Sampietro, E.N.; Quiroga, E.N.; Vattuone, M.A. Isolation of antibacterial components from infusion of Caesalpinia paraguaiensis bark. A bio-guided phytochemical study. Food Chem. 2011, 126, 395–404. [Google Scholar]
- Vattuone, M.A.; Martínez, R.H.; Corzo, A.G. Actividad antibacteriana de extractos de hojas de Caesalpinia paraguaiensis, Par Burk, “Guayacán”. Quebracho 2008, 15, 37–41. [Google Scholar]
- Lee, H.; Lim, M.; Jeon, J.; Jeong, E.; Lee, C. Antimicrobial activity of 5-hidroxy-1,4-naphtoquinine isolated from Caesalpinia sappan toward intestinal bacteria. Food Chem. 2007, 100, 1254–1258. [Google Scholar]
- Jeong, G.S.; Lee, D.S.; Kwon, T.; Lee, H.; An, R.; Kim, Y. Cytoprotective constituents of the heartwood of Caesalpinia sappan on glutamate-induced oxidative damage in HT22 cells. Biol. Pharm. Bull. 2009, 32, 945–949. [Google Scholar] [CrossRef]
- Lee, Y.M.; Jeong, G.S.; Lim, H.D.; An, R.B.; Kim, Y.C.; Kim, E.C. Isoliquiritigenin 2'-methyl ether induces growth inhibition and apoptosis in oral cancer cells via hemeoxygenase-1. Toxicol. In Vitro 2010, 24, 776–782. [Google Scholar]
- Choi, B.M.; Lee, J.; Gao, S.S.; Eun, S.Y.; Kim, Y.; Ryu, S.; Choi, Y.; Park, R.; Kwon, D.Y.; Kim, B. Brazilin and the extract from Caesalpinia sappan L. protect oxidative injury through the expression on heme oxygenase-1. BioFactor 2007, 30, 149–157. [Google Scholar] [CrossRef]
- Du, L.; Xing, D.; Su, H.; Lin, H.; Zhang, H.; Shen, J. Brazilein protects the brain against focal cerebral ischemia reperfusion injury correlating to inflammatory response suppression. Eur. J. Pharmacol. 2007, 558, 88–95. [Google Scholar] [CrossRef]
- Yen, C.; Nakagawa-Got, K.; Hwang, T.; Wu, P.; Morris-Natschke, S.L.; Lai, W.; Bastow, K.F.; Chang, F.; Wu, Y.; Lee, K. Antitumor agents. 27: Total synthesis and evaluation of brazilein and analogs as anti-inflammatory and cytotoxic agents. Bioorg. Med. Chem. Lett. 2010, 20, 1037–1039. [Google Scholar]
- Nakamura, S.E.; Kurosaki, F.; Arisawa, M.; Mukainaka, T.; Okuda, M.; Tokuda, H.; Nishino, H.; Pastore, F.J. Cancer chemopreventive effects of constituents of Caesalpinia ferrea and related compounds. Cancer Lett. 2002, 177, 119–124. [Google Scholar] [CrossRef]
- Bacchi, E.M.; Sertié, J.A.A. Antiulcer action of Styrax camporum and Caesalpinia ferrea in rats. Planta Med. 1994, 60, 118–120. [Google Scholar] [CrossRef]
- Bacchi, E.M.; Sertié, J.A.A.; Villa, N.; Katz, H. Antiulcer action and toxicity of Styrax camporum and Caesalpinia ferrea. Planta Med. 1995, 61, 204–207. [Google Scholar] [CrossRef]
- Jiang, R.W.; Ma, S.; But, P.P.; Mak, T.C.W. New antiviral cassane furanoditerpenes from Caesalpinia minax. J. Nat. Prod. 2001, 64, 1266–1272. [Google Scholar]
- Jiang, T.; But, P.P.H.; Ma, S.; Ye, W.; Chan, S.; Mak, T.C.W. Structure and antiviral properties of macrocaesalmin, a novel cassane furanoditerpenoid lactone from the seeds of Caesalpinia minax Hance. Tetrahedron Lett. 2002, 43, 2415–2418. [Google Scholar]
- Hong, C.H.; Hur, S.K.; Oh, O.J.; Kim, S.S.; Nam, K.A.; Lee, S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J. Ethnopharmacol. 2002, 83, 153–159. [Google Scholar] [CrossRef]
- Jun, H.; Xiaoling, Y.; Wei, W.; Hao, W.; Lei, H.; Li, J.D. Antioxidant activity in vitro of three constituents from Caesalpinia sappan L. Tsinghua Sci. Technol. 2008, 13, 474–479. [Google Scholar] [CrossRef]
- Sampaio, F.C.; Pereira, M.S.V.; Dias, C.S.; Costa, V.C.O.; Conde, N.C.O.; Buzalaf, M.A.R. In vitro antimicrobial activity of Caesalpinia ferrea Martius fruits against oral pathogens. J. Ethnopharmacol. 2009, 124, 289–294. [Google Scholar] [CrossRef]
- Menezes, I.A.C.; Moreira, I.J.A.; Carvalho, A.A.; Antoniolli, A.R.; Santos, M.R.V. Cardiovascular effects of the aqueous extract from Caesalpinia ferrea: Involvement of ATP-sensitive potassium channels. Vascul. Pharmacol. 2007, 47, 41–47. [Google Scholar] [CrossRef]
- Hu, L.; Liu, G.; Ma, L.; Li, D. Cassane diterpene-lactones from the seed of Caesalpinia minax Hance. Chem. Biodiv. 2006, 3, 1260–1265. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Karalai, C.; Ponglimanont, C.; Tewthkul, S.; Chantrapromma, C. Potential anti-inflammatory diterpenoids from the roots of Caesalpinia mimosoides Lamk. Phytochemistry 2010, 71, 1756–1764. [Google Scholar]
- Srinivasan, R.; Chandrasekar, M.J.N.; Nanjan, M.J.; Suresh, B. Antioxidant activity of Caesalpinia digyna root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef]
- Cavalheiro, M.G.; Farias, D.F.; Fernandes, G.S.; Nnunes, E.P.; Cavalcanti, F.S.; Vasconcelos, I.M.; Melo, V.M.M.; Ccarvalho, A.F.U. Atividades biológicas e enzimáticas do extrato aquoso de sementes de Caesalpinia ferrea Mart., Leguminosae. Rev. Bras. Farmacogn. 2009, 19, 586–591. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Srisuwan, R.; Karalai, C.; Ponglimanont, C.; Chantrapromma, S.; Chantrapromma, K.; Fun, H.; Anjum, S.; Rahman, A. New diterpenoids from stems and roots of Caesalpinia crista. Tetrahedron 2005, 61, 8656–8662. [Google Scholar] [CrossRef]
- Laekeman, G.M.; Kuria, K.A.M.; Coster, S.; Muriuki, G.; Masengo, W.; Kibwage, I.; Hoogmartens, J. Antimalarial activity of Ajuda remota Benth (Labiatae) and Caesalpinia volkensii Harms (Caesalpiniaceae): In vitro confirmation of ethnopharmacological use. J. Ethnopharmacol. 2001, 74, 141–148. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Anagnostopulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimepoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar]
- Arif, T.; Mandal, T.K.; Kumar, N.; Bhosale, J.D.; Hole, A.; Sharma, G.L.; Padhi, M.M.; Lavekar, G.S.; Dabur, R. In vitro and in vivo antimicrobial activities of seeds of Caesalpinia bonduc (Lin.) Roxb. J. Ethnopharmacol. 2009, 123, 177–180. [Google Scholar]
- Zhang, Q.; Liu, X.; Liang, J.; Min, Z. Chemical constituents from the stem of Caesalpinia decapetala. Chin. J. Nat. Med. 2008, 6, 168–172. [Google Scholar] [CrossRef]
- Rao, V.C.; Damu, G.L.V.; Sudhakar, D.; Siddiah, V.; Rao, V.M. New efficient synthesis and bioactivity of homoisoflavonoids. Arkivoc 2008, 11, 285–294. [Google Scholar]
- Mandal, S.; Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, N. Assessment of the antioxidant and reactive oxygen species scavening activity of methanolic extract of Caesalpinia crista leaf. eCAM 2009. [Google Scholar] [CrossRef]
- Bulbovas, P.; Moraes, R.M.; Rinaldi, M.C.S.; Cunha, A.L.; Delitti, W.B.C.; Domingos, M. Leaf antioxidant fluctuations and growth responses in saplings of Caesalpinia echinata Lam. (brazilwood) under an urban stressing environment. Ecotoxicol. Environ. Saf. 2010, 73, 664–670. [Google Scholar] [CrossRef]
- Kannur, D.M.; Hukkeri, V.I.; Akki, K.S. Antidiabetic activity of Caesalpinia bonducella seed extracts in rats. Fitoterapia 2006, 77, 546–549. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Biswas, T.K.; Rokeya, B.; Ali, L.; Mosihuzzaman, M.; Nahar, N.; Khan, A.K.A.; Mukherjee, B. Advanced studies on the hypoglycemic effect of Caesalpinia bonducella F in type 1 and 2 diabetes in Long Evans rats. J. Ethnopharmacol. 2003, 84, 41–46. [Google Scholar] [CrossRef]
- Kannur, D.M.; Hukkeri, V.I.; Akki, K.S. Adaptogenic activity of Caesalpinia bonduc seed extracts in rats. J. Ethnopharmacol. 2006, 108, 327–331. [Google Scholar] [CrossRef]
- Muruganantham, N.; Basavaraj, K.H.; Dhanabal, S.P.; Praveen, T.K.; Shamasundar, N.M.; Rao, K.S. Screening of Caesalpinia bonduc leaves for antipsoriatic activity. J. Ethnopharmacol. 2011, 133, 897–901. [Google Scholar] [CrossRef]
- Yadav, P.P.; Arora, A.; Bid, H.K.; Konwar, R.T.; Kanojiva, S. New cassane butenolide hemiketal diterpenes from the marine creeper Caesalpinia bonduc and their proliferative activity. Tetrahedron Lett. 2007, 48, 7194–7198. [Google Scholar]
- Yadav, P.P.; Maurya, R.; Sarkar, J.; Arora, A.; Kanojiya, S.; Sinha, S.; Srivasava, M.N.; Raghubir, R. Cassane diterpenes from Caesalpinia bonduc. Phytochemistry 2009, 70, 256–261. [Google Scholar]
- Saravanan, K.S.; Saraswathy, G.R.; Periyanayagam, K.; Ismail, M.; Betanabhatla, K.S.; Athimoolam, J. In vitro antifilarial activity of Caesalpinia bonduc (l) Roxb. against microfilariae of Wuchereria bancrofti and macrofilariae of Setaria digitata. Pharmacologyonline 2008, 2, 550–559. [Google Scholar]
- Zamblé, A.; Martin-Nizard, F.; Sahpaz, S.; Hennebelle, T.; Staels, B.; Bordet, R.; Duriez, P.; Brunet, C.; Bailleul, F. Vasoactivity, antioxidant and aphrodisiac properties of Caesalpinia benthamiana roots. J. Ethnopharmacol. 2008, 116, 112–119. [Google Scholar] [CrossRef]
- Moon, H.; Chung, I.; Seo, S.; Kang, E. Protective Effects of 3'-deoxy-4-O-methylepisappanol from Caesalpinia sappan against glutamate-induced neurotoxicity in primary cultured rat cortical cells. Phytother. Res. 2010, 24, 463–465. [Google Scholar] [CrossRef]
- Jiang, R.; But, P.P.H.; Ma, S.; Ma, T.C.W. Furanoditerpenoid lactones from the seeds of Caesalpinia minax Hance. Phytochemistry 2001, 57, 517–521. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Tran, Q.L.; Kadota, S. Neosappanone, A, a xanthine oxidase (XO) inhibitory dimeric methanodibenzoxocinone with a new carbon skeleton form Caesalpinia sappan. Tetrahedron Lett. 2004, 45, 8519–8522. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zanin, J.L.B.; De Carvalho, B.A.; Salles Martineli, P.; Dos Santos, M.H.; Lago, J.H.G.; Sartorelli, P.; Viegas, C., Jr.; Soares, M.G. The Genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and Pharmacological Characteristics. Molecules 2012, 17, 7887-7902. https://doi.org/10.3390/molecules17077887
Zanin JLB, De Carvalho BA, Salles Martineli P, Dos Santos MH, Lago JHG, Sartorelli P, Viegas C Jr., Soares MG. The Genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and Pharmacological Characteristics. Molecules. 2012; 17(7):7887-7902. https://doi.org/10.3390/molecules17077887
Chicago/Turabian StyleZanin, João L. Baldim, Bianca A. De Carvalho, Paloma Salles Martineli, Marcelo Henrique Dos Santos, João Henrique G. Lago, Patrícia Sartorelli, Cláudio Viegas, Jr., and Marisi G. Soares. 2012. "The Genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and Pharmacological Characteristics" Molecules 17, no. 7: 7887-7902. https://doi.org/10.3390/molecules17077887
APA StyleZanin, J. L. B., De Carvalho, B. A., Salles Martineli, P., Dos Santos, M. H., Lago, J. H. G., Sartorelli, P., Viegas, C., Jr., & Soares, M. G. (2012). The Genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and Pharmacological Characteristics. Molecules, 17(7), 7887-7902. https://doi.org/10.3390/molecules17077887



