Tebrophen — An Old Polyphenol Drug with Anticancer Potential †
Abstract
:Abbreviations
| BDE | bond dissociation enthalpy |
| HAT | hydrogen atom transfer |
| HTS | high-throughput screening |
| IP | ionization potential |
| PDs | population doublings |
| PMA | phorbol-12-myristate acetate |
| RSA | radical scavenging activity |
| SET | single electron transfer |
| TEB | tebrophen |
1. Introduction

2. Results
2.1. Docking Pose of Tebrophen within DPPIV Catalytic Site
2.2. In Vitro Inhibition of ZAP-70, Lck kinase and DPPIV


2.3. Oxidative Burst
2.4. Cytotoxicity Assay
2.5. The Effects of Tebrophenon on Cell Cultures Proliferation
2.5.1. Normal Human Skin Fibroblasts NF (Primary Cells)

2.5.2. Neonatal Foreskin Fibroblasts MJ90 (Primary Cells)

2.5.3. Breast Adenocarcinoma MDA-MB-231 Cell Line


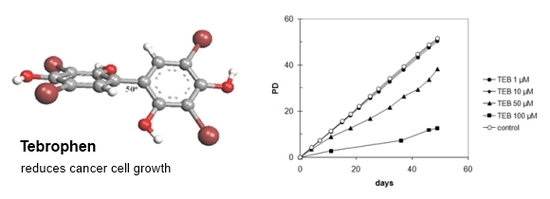
2.5.4. Strong Inhibitory Effect of Tebrophen on Proliferation of Prostate PC3 Cell Line

2.5.5. Bone Osteosarcoma U2OS Cell Line

2.5.6. Cervical Adenocarcinoma HeLa Cell Line

2.6. Radical Scavenging Activity of Tebrophen


| Species | BDE | IP | Acidity | ABTS+ assay (TEAC, mM) b |
|---|---|---|---|---|
| ortho-Tebrophen | 82.37 | 169.6 | 326.3 | NA |
| para-Tebrophen | 81.98 | 169.6 | 324.9 | NA |
| Apigenin | 82.2 | 176.0 | 321.3 | 0.086 |
| Kaempferol | 80.9 | 168.0 | 322.7 | 1.59 |
| Luteolin | 74.5 | 174.4 | 2.18 | |
| Quercetin | 72.3 | 166.1 | 316.5 | 4.42 |
| Resveratrol | 77.3 | 161.3 | 327.5 | 2.14 |
3. Discussion
4. Experimental
4.1. Tebrophen
4.2. In Silico
4.2.1. Molecular Docking
4.2.2. Quantum-Chemical Calculations
4.3. In Vitro ZAP-70 and Lck Kinase, and DPPIV Inhibition Protocols
4.4. Cytotoxicity Assay
4.5. Oxidative Burst Assay
4.6. Functional Assays
4.6.1. Cell Culture
4.6.2. SA-β-galactosidase Staining and 3H-Thymidine Labeling Index
4.6.3. DiI Staining and Flow Cytometry
5. Conclusions
Conflict of Interest
Acknowledgements
- Sample Availability: Sample of the compound is available from the authors.
References
- Jelić, D.; Mesar, V.; Bašic, I.; Nadramija, D.; Verbanac, D. Novel approach to drug discovery in PLIVA-establishing of HTS Unit and unique Compounds Library. Pharmachem 2003, 2, 64–67. [Google Scholar]
- Jelić, D.; Toth, T.; Verbanac, D. Macromolecular Databases—A background of bioinformatics. Food Technol. Biotech. 2003, 41, 269–286. [Google Scholar]
- Jelić, D.; Nujić, K.; Stepanić, V.; Kovačević, K.; Verbanac, D. 6-Imino-2-thioxo-pyrimidinones as a new class of dipeptidyl peptidase IV inhibitors. Med. Chem. Res. 2011, 20, 339–345. [Google Scholar] [CrossRef]
- Verbanac, D.; Jelić, D.; Stepanić, V.; Tatić, I.; Žiher, D.; Koštrun, S. Combined in silico and in vitro approach to drug screening. Croat. Chem. Acta 2005, 78, 133–139. [Google Scholar]
- Thompson, M.A.; Ohnuma, K.; Abe, M.; Morimoto, C.; Dang, N.H. CD26/dipeptidyl peptidase IV as a novel therapeutic target for cancer and immune disorders. Mini Rev. Med. Chem. 2007, 7, 253–273. [Google Scholar] [CrossRef]
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596. [Google Scholar]
- Cooke, M.P.; Abraham, K.M.; Forbush, K.A.; Perlmutter, R.M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell 1991, 65, 281–291. [Google Scholar] [CrossRef]
- Davidson, D.; Chow, L.M.; Fournel, M.; Veillette, A. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J. Exp. Med. 1992, 175, 1483–1492. [Google Scholar] [CrossRef]
- Wange, R.L.; Samelson, L.E. Complex complexes: Signaling at the TCR. Immunity 1996, 5, 197–205. [Google Scholar] [CrossRef]
- Samelson, L.E.; Davidson, W.F.; Morse, H.C.; Klausner, R.D. Abnormal tyrosine phosphorylation on T-cell receptor in lymphoproliferative disorders. Nature 1986, 324, 674–676. [Google Scholar]
- Hamblin, T.J. Predicting Progression-ZAP-70 in CLL. N. Engl. J. Med. 2004, 351, 856–857. [Google Scholar] [CrossRef]
- Parnham, M.J.; Verbanac, D. Mild Plant and Dietary Immunomodulators. In Principles of Immunopharmacology; Nijkamp, F.P., Parnham, M.J., Eds.; Springer: Basel, Switzerland, 2011; pp. 451–469. [Google Scholar]
- Čalić, M.; Jelić, D.; Antolović, R.; Nujić, K.; Marjanović, N.; Stupin-Polančec, D.; Vikić-Topić, S.; Verbanac, D. Flavonoids as inhibitors of lck and fyn kinases. Croat. Chem. Acta 2005, 78, 367–374. [Google Scholar]
- Jelić, D.; Mildner, B.; Koštrun, S.; Nujić, K.; Verbanac, D.; Čulić, O.; Antolović, R.; Brandt, W. Homology modeling of human Fyn kinase structure: discovery of rosmarinic acid as a new Fyn kinase inhibitor and in silico study of its possible binding modes. J. Med. Chem. 2007, 50, 1090–1100. [Google Scholar] [CrossRef]
- Boonacker, E.; Van Noorden, C.J. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 2003, 82, 53–73. [Google Scholar] [CrossRef]
- Sato, K.; Dang, N.H. CD26: A novel treatment target for T-cell lymphoid malignancies? Int. J. Oncol. 2003, 22, 481–497. [Google Scholar]
- Ohnuma, K.; Dang, N.H.; Morimoto, C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008, 29, 295–301. [Google Scholar] [CrossRef]
- Havre, P.A.; Abe, M.; Urasaki, Y.; Ohnuma, K.; Morimoto, C.; Dang, N.H. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. 2008, 13, 1634–1645. [Google Scholar] [CrossRef]
- Pratley, R.E.; Salsali, A. Inhibition of DPP-4: A new therapeutic approach for the treatment of type 2 diabetes. Curr. Med. Res. Opin. 2007, 23, 919–931. [Google Scholar] [CrossRef]
- Pro, B.; Dang, N.H. CD26/dipeptidyl peptidase IV and its role in cancer. Histol. Histopathol. 2004, 19, 1345–1351. [Google Scholar]
- Deacon, C.F.; Carr, R.D.; Holst, J.J. DPP-4 inhibitor therapy: New directions in the treatment of type 2 diabetes. Front. Biosci. 2008, 13, 1780–1794. [Google Scholar] [CrossRef]
- Wiedeman, P.E. DPPIV inhibition: Promising therapy for the treatment of type 2 diabetes. Prog. Med. Chem. 2007, 45, 63–109. [Google Scholar] [CrossRef]
- Mentlein, R.; Dahms, P.; Grandt, D.; Kruger, R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Pept. 1993, 49, 133–144. [Google Scholar] [CrossRef]
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)—Role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24. [Google Scholar] [CrossRef]
- Bansal, P.; Paul, P.; Mudgal, J.; Nayak, G.; Thomas, P.S.; Priyadarsini, K.I.; Unnikrishnan, M.K. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp. Toxicol. Pathol. 2011, in press.. [Google Scholar]
- Rasmussen, H.B.; Branner, S.; Wiberg, F.C.; Wagtmann, N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat. Struct. Biol. 2003, 10, 19–25. [Google Scholar] [CrossRef]
- Andreotti, P.E.; Ludwig, G.V.; Peruski, A.H.; Tuite, J.J.; Morse, S.S.; Peruski, L.F., Jr. Immunoassay of infectious agents. Biotechniques 2003, 35, 850–859. [Google Scholar]
- Crowther, J.R. ELISA: Theory and practice; Humana Press Inc.: Totowa, NJ, USA, 1995; pp. 1–218. [Google Scholar]
- Wilson, E.; Olcott, M.C.; Bell, R.M.; Merrill, A.H., Jr.; Lambeth, J.D. Inhibition of the oxidative burst in human neutrophils by sphingoid long-chain bases. Role of protein kinase C in activation of the burst. J. Biol. Chem. 1986, 261, 12616–12623. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ferenac, M.; Polančec, D.; Huzak, M.; Pereira-Smith, O.M.; Rubelj, I. Early-senescing human skin fibroblasts do not demonstrate accelerated telomere shortening. J. Gerontol. A-Biol. 2005, 60, 820–829. [Google Scholar] [CrossRef]
- Vidaček, N.S.; Čukušić, A.; Ivanković, M.; Fulgosi, H.; Huzak, M.; Smith, J.R.; Rubelj, I. Abrupt telomere shortening in normal human fibroblasts. Exp. Gerontol. 2010, 45, 235–242. [Google Scholar] [CrossRef]
- Brinkley, B.R.; Beall, P.T.; Wible, L.J.; Mace, M.L.; Turner, D.S.; Cailleau, R.M. Variations in cell form and cytoskeleton in human breast carcinoma cells in vitro. Cancer Res. 1980, 40, 3118–3129. [Google Scholar]
- Čukušić, A.; Ivanković, M.; Škrobot, N.; Ferenac, M.; Gotić, I.; Matijašić, M.; Polančec, D.; Rubelj, I. Spontaneous senescence in the MDA-MB-231 cell line. Cell Prolif. 2006, 39, 205–216. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 1979, 17, 16–23. [Google Scholar]
- Ponten, J.; Saksela, E. Two established in vitro cell lines from human mesenchymal tumours. Int. J. Cancer 1967, 2, 434–447. [Google Scholar] [CrossRef]
- Ivanković, M.; Čukušić, A.; Gotić, I.; Škrobot, N.; Matijašić, M.; Polančec, D.; Rubelj, I. Telomerase activity in HeLa cervical carcinoma cell line proliferation. Biogerontology 2007, 8, 163–172. [Google Scholar] [CrossRef]
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 1953, 97, 695–710. [Google Scholar] [CrossRef]
- Čukušić, A.; Škrobot, V.N.; Sopta, M.; Rubelj, I. Telomerase regulation at the crossroads of cell fate. Cytogenet. Genome Res. 2008, 122, 263–272. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar]
- Robles, S.J.; Adami, G.R. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 1998, 16, 1113–1123. [Google Scholar]
- Roninson, I.B. Tumor cell senescence in cancer treatment. Cancer Res. 2003, 63, 2705–2715. [Google Scholar]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Benitez, D.A.; Pozo-Guisado, E.; Alvarez-Barrientos, A.; Fernandez-Salguero, P.M.; Castellon, E.A. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J. Androl. 2007, 28, 282–293. [Google Scholar]
- Chen, D.; Chen, M.S.; Cui, Q.C.; Yang, H.; Dou, Q.P. Structure-proteasome-inhibitory activity relationships of dietary flavonoids in human cancer cells. Front. Biosci. 2007, 12, 1935–1945. [Google Scholar] [CrossRef]
- Knowles, L.M.; Zigrossi, D.A.; Tauber, R.A.; Hightower, C.; Milner, J.A. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr. Cancer 2000, 38, 116–122. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Amić, D.; Lučić, B. Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorg. Med. Chem. 2010, 18, 28–35. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar]
- Zhang, M.; Qiao, Y.; Suo, Z. Correlation of DPPIV expression with clinicopathological features and prognosis in epithelial ovarian carcinoma. Zhonghua Zhong Liu Za Zhi 2008, 30, 848–852. [Google Scholar]
- Gonzalez-Angulo, A.M.; Meric-Bernstam, F. Metformin: A therapeutic opportunity in breast cancer. Clin. Cancer Res. 2010, 16, 1695–1700. [Google Scholar] [CrossRef]
- Janjetović, K.; Vučićević, L.; Misirkić, M.; Vilimanovich, U.; Tovilović, G.; Zogović, N.; Nikolić, Z.; Jovanović, S.; Bumbasirević, V.; Trajković, V.; et al. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through AMPK-independent activation of Akt. Eur. J. Pharmacol. 2011, 651, 41–50. [Google Scholar] [CrossRef]
- Deindl, S.; Kadlecek, T.A.; Brdicka, T.; Cao, X.; Weiss, A.; Kuriyan, J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell 2007, 129, 735–746. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Kang, N.J.; Shin, S.H.; Lee, H.J.; Lee, K.W. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol. Ther. 2011, 130, 310–324. [Google Scholar] [CrossRef]
- Stel’makh, S.G.; Levchenko, I.F. Experience in the use of Tebrophen for influenza prevention. Zh. Mikrobiol. Epidemiol. Immunobiol. 1975, 6, 113–115. [Google Scholar]
- Rubelj, I.; Huzak, M.; Brdar, B. Sudden senescence syndrome plays a major role in cell culture proliferation. Mech. Ageing Dev. 2000, 112, 233–241. [Google Scholar] [CrossRef]
- Basu, S.; Tindall, D.J. Androgen action in prostate cancer. Horm. Cancer 2010, 1, 223–228. [Google Scholar] [CrossRef]
- Zheng, F.L.; Ban, S.R.; Feng, X.E.; Zhao, C.X.; Lin, W.; Li, Q.S. Synthesis and in vitro protein tyrosine kinase inhibitory activity of furan-2-yl(phenyl)methanone derivatives. Molecules 2011, 16, 4897–4911. [Google Scholar] [CrossRef]
- Rarey, M.; Kramer, B.; Lengauer, T.; Klebe, G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996, 261, 470–489. [Google Scholar] [CrossRef]
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.; Meyer, E.F., Jr.; Brice, M.D.; Rodgers, J.R.; Kennard, O.; Shimanouchi, T.; Tasumi, M. The Protein Data Bank: A computer-based archival file for macromolecular structures. J. Mol. Biol. 1977, 112, 535–542. [Google Scholar] [CrossRef]
- Sadowski, J.; Kubinyi, H. A scoring scheme for discriminating between drugs and nondrugs. J. Med. Chem. 1998, 41, 3325–3329. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Gaussian, Inc.: Wallingford, CT, USA, 2004.
- Koegl, M.; Kypta, R.M.; Bergman, M.; Alitalo, K.; Courtneidge, S.A. Rapid and efficient purification of Src homology 2 domain-containing proteins: Fyn, Csk and phosphatidylinositol 3-kinase p85. Biochem. J. 1994, 302, 737–744. [Google Scholar]
- Ramer, S.E.; Winkler, D.G.; Carrera, A.; Roberts, T.M.; Walsh, C.T. Purification and initial characterization of the lymphoid-cell protein-tyrosine kinase p56lck from a baculovirus expression system. Proc. Natl. Acad. Sci. USA 1991, 88, 6254–6258. [Google Scholar]
- Watts, J.D.; Wilson, G.M.; Ettenhadieh, E.; Clark-Lewis, I.; Kubanek, C.A.; Astell, C.R.; Marth, J.D.; Aebersold, R. Purification and initial characterization of the lymphocyte-specific protein-tyrosyl kinase p56lck from a baculovirus expression system. J. Biol. Chem. 1992, 267, 901–907. [Google Scholar]
- Bovaird, J.H.; Ngo, T.T.; Lenhoff, H.M. Optimizing the o-phenylenediamine assay for horseradish peroxidase: Effects of phosphate and pH, substrate and enzyme concentrations, and stopping reagents. Clin. Chem. 1982, 28, 2423–2426. [Google Scholar]
- Rubelj, I.; Huzak, M.; Brdar, B.; Pereira-Smith, O.M. A single-stage mechanism controls replicative senescence through Sudden Senescence Syndrome. Biogerontology 2002, 3, 213–222. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rubelj, I.; Stepanić, V.; Jelić, D.; Vidaček, N.Š.; Kalajžić, A.Ć.; Ivanković, M.; Nujić, K.; Matijašić, M.; Verbanac, D. Tebrophen — An Old Polyphenol Drug with Anticancer Potential †. Molecules 2012, 17, 7864-7886. https://doi.org/10.3390/molecules17077864
Rubelj I, Stepanić V, Jelić D, Vidaček NŠ, Kalajžić AĆ, Ivanković M, Nujić K, Matijašić M, Verbanac D. Tebrophen — An Old Polyphenol Drug with Anticancer Potential †. Molecules. 2012; 17(7):7864-7886. https://doi.org/10.3390/molecules17077864
Chicago/Turabian StyleRubelj, Ivica, Višnja Stepanić, Dubravko Jelić, Nikolina Škrobot Vidaček, Andrea Ćukušić Kalajžić, Milena Ivanković, Krunoslav Nujić, Mario Matijašić, and Donatella Verbanac. 2012. "Tebrophen — An Old Polyphenol Drug with Anticancer Potential †" Molecules 17, no. 7: 7864-7886. https://doi.org/10.3390/molecules17077864
APA StyleRubelj, I., Stepanić, V., Jelić, D., Vidaček, N. Š., Kalajžić, A. Ć., Ivanković, M., Nujić, K., Matijašić, M., & Verbanac, D. (2012). Tebrophen — An Old Polyphenol Drug with Anticancer Potential †. Molecules, 17(7), 7864-7886. https://doi.org/10.3390/molecules17077864