Biochemical Characterization of an In-House Coccidioides Antigen: Perspectives for the Immunodiagnosis of Coccidioidomycosis
Abstract
:1. Introduction
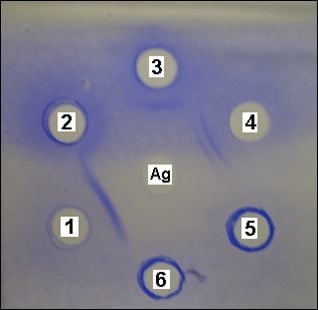
2. Results and Discussion
| Protein | Species | Amino acid sequence | % Similarity | Accession number |
|---|---|---|---|---|
| 45–67 kDa band (this report) | Coccidioides posadasii | 1AGKLGFALGVKNADGSCKS19 | ||
| β-Glucosidase * | Coccidioides posadasii | 20AGKLGFALGVKNADGSCKS38 | 100 | XP_003070157 |
| 67–97 kDa band (this report) | Coccidioides posadasii | 1NAHYVLAAIVALGWRG16 | ||
| Glutamine synthetase * | Coccidioides posadasii | 403NAHYVLAAIVALGWRG418 | 100 | XP_003072037 |
3. Experimental
3.1. Selection of the Fungal Strains
3.2. Obtaining and Fractionating the Protein Antigen
3.3. Evaluation of the Antigen Immunoreactivity by the Double Radial Immunodiffusion Technique (Ouchterlony)
3.4. Evaluation of the Antigen Immunoreactivity by the Western Blot Technique
3.5. Biochemical Analysis
3.5.1. Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.5.2. Determination of N-Terminal Sequencing
4. Conclusions
Acknowledgements
- Sample Availability: Not available.
References and Notes
- Ramani, R.; Chatuverdi, V. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathologia 2007, 163, 315–319. [Google Scholar] [CrossRef]
- Muñoz-Hernández, B.; Martínez-Rivera, M.A.; Cortés, G.P.; Tapia-Díaz, A.; Zavala, M.E.M. Mycelial forms of Coccidioides spp. in the parasitic phase associated to pulmonary coccidioidomycosis with type 2 diabetes mellitus. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 813–820. [Google Scholar] [CrossRef]
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of Coccidioidomycosis with use of the Coccidioides Antigen Enzyme Immunoassay. Clin. Infect. Dis. 2008, 47, 69–73. [Google Scholar] [CrossRef]
- Saubolle, M.A.; McKellar, P.P.; Sussland, D. Epidemiologic, Clinical, and Diagnostic Aspects of Coccidioidomycosis. J. Clin. Microbiol. 2007, 45, 26–30. [Google Scholar] [CrossRef]
- Castañon-Olivares, L.C.; Güerena-Elizalde, D.; Gonzalez-Martinez, M.R.; Licea-Navarro, A.F.; Gonzalez-Gonzalez, G.M.; Aroch-Calderon, A. Molecular Identification of Coccidioides Isolates from Mexican Patients. Ann. NY Acad. Sci. 2007, 1111, 326–335. [Google Scholar] [CrossRef]
- Pappagianis, D.; Zimmer, B.L. Serology of coccidioidomycosis. Clin. Microbiol. Rev. 1990, 3, 247–268. [Google Scholar]
- Pappagianis, D. Seeking a vaccine against Coccidioides immitis and serologic studies: Expectations and realities. Fungal Genet. Biol. 2001, 32, 1–9. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Cordeiro, R.A.; Rocha, M.F.G.; Fechine, M.A.B.; Furtado, F.M.; Nagao-Dias, M.A.T.; Camargo, Z.P.; Sidrim, J.J.C. Coccidioidal pericarditis: A rapid presumptive diagnosis by an in-house antigen confirmed by mycological and molecular methods. J. Med. Microbiol. 2008, 57, 1288–1292. [Google Scholar] [CrossRef]
- Wheat, J.L. Approach to the diagnosis of the endemic mycoses. Clin. Chest. Med. 2009, 30, 373–389. [Google Scholar]
- Kirkland, T.N.; Thomas, P.W.; Finley, F.; Cole, G.T. Immunogenicity of a 48-Kilodalton recombinant T-cell-reactive protein of Coccidioides immitis. Gene 1998, 66, 424–431. [Google Scholar]
- Blair, J.E.; Coakley, B.; Santelli, A.C.; Hentz, J.G.; Wengenack, N.L. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 2006, 162, 317–324. [Google Scholar] [CrossRef]
- Zacharius, R.M.; Zen, T.E.; Morrison, J.H.; Woodlock, J.J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal. Biochem. 1969, 30, 148–152. [Google Scholar]
- Cox, R.A.; Britt, L.A. Isolation and identification of an exoantigen specific for Coccidioides immitis. Infect. Immun. 1986, 52, 138–143. [Google Scholar]
- Yuan, L.; Cole, G.T. Isolation and characterization of an extracellular proteinase of Coccidioides immitis. Infect. Immun. 1987, 55, 1970–1978. [Google Scholar]
- Elias-Costa, M.R.I. Obtención de un nuevo antigen del Coccidioides immitis y desarrollo de un modelo experimental de enfermedad en ratas Wistar. Rev. Argent. Microbiol. 1995, 18, 3–29. [Google Scholar]
- Kim, S.Y.; Shin, A.R.; Kim, H.J.; Cho, S.N.; Park, J.K.; Shin, S.J. Identification of Rv2041c, a novel immunogenic antigen from Mycobacterium tuberculosis with serodiagnostic potential. Scand. J. Immunol. 2009, 70, 457–464. [Google Scholar] [CrossRef]
- Johnson, S.M.; Simmons, K.A.; Pappagianis, D. Amplification of coccidioidal DNA in clinical specimens by PCR. J. Clin. Microbiol. 2004, 42, 1982–1985. [Google Scholar] [CrossRef]
- Cordeiro, R.A.; Fechine, M.A.B.; Brilhante, R.S.N.; Rocha, M.F.G.; Costa, A.K.F.; Nagao-Dias, M.A.T.; Camargo, Z.P.; Sidrim, J.J.C. Serologic Detection of Coccidioidomycosis Antibodies in Northeast Brazil. Mycopathologia 2008, 167, 187–190. [Google Scholar]
- Klein, B.S.; Jones, J.M. Isolation, purification, and radiolabeling of a novel 120-kD surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. J. Clin. Invest. 1990, 85, 152–161. [Google Scholar] [CrossRef]
- Pappagianis, D. Serologic studies in coccidioidomycosis. Semin. Respir. Infect. 2001, 16, 242–250. [Google Scholar] [CrossRef]
- Zimmer, B.L.; Pappagianis, D. Comparison of immunoblot analyses of spherule-endospore-phase extracellular protein and mycelial-phase antigen of Coccidioides immitis. Infect. Immun. 1986, 53, 64–70. [Google Scholar]
- Thomas, P.W.; Wyckoff, E.E.; Pishko, E.J.; Yu, J.J.; Kirkland, T.N.; Cole, G.T. The hsp60 gene of the human pathogenic fungus Coccidioides immitis encodes a T-cell reactive protein. Gene 1997, 199, 83–91. [Google Scholar] [CrossRef]
- Yu, J.J.; Smithson, S.L.; Thomas, P.W.; Kirkland, T.N.; Cole, G.T. Isolation and characterization of the urease gene (URE) from the pathogenic fungus Coccidioides immitis. Gene 1997, 198, 387–391. [Google Scholar] [CrossRef]
- Jeng, W.Y.; Wang, N.C.; Lin, M.H.; Lin, C.T.; Liawb, Y.C.; Chang, W.J.; Liu, C.I.; Liang, P.H.; Wang, A.H.J. Structural and functional analysis of three β-glucosidases from bacterium Clostridium cellulovorans, fungus Trichoderma reesei and termite Neotermes koshunensis. J. Struct. Biol. 2011, 173, 46–56. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Azevedo, T.R.; Terra, W.R.; Ferreira, F. Purification and characterization of three β-glycosidases from midgut of the sugar cane borer, Diatraea saccharalis. Insect Biochem. Mol. Biol. 2003, 33, 81–92. [Google Scholar] [CrossRef]
- Morant, A.V.; Jørgensen, K.; Jørgensen, C.; Paquette, S.M.; Sánchez-Pérez, R.; Møller, B.L.; Bak, S. β-Glucosidases as detonators of plant chemical defense. Phytochemistry 2008, 69, 1795–1813. [Google Scholar]
- Gargouri, M.; Smaali, I.; Maugard, T.; Legoy, M.D.; Marzouki, N. Fungus β-glycosidases: Immobilization and use in alkyl-β-glycoside synthesis. J. Mol. Catal. B Enzym. 2004, 29, 89–94. [Google Scholar] [CrossRef]
- Hung, C.Y.; Yu, J.J.; Lehmann, P.F.; Cole, G.T. Cloning and Expression of the Gene Which Encodes a Tube Precipitin Antigen and Wall-Associated β-Glucosidase of Coccidioides immitis. Infect. Immun. 2001, 69, 2211–2222. [Google Scholar]
- Herrmann, P.S.P.; Fromme, R.J.; Colnago, L.A. Análise da estrutura da glutamina sintetase por microscopia de força atômica: Primeiros resultados. Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA), 1997. Available online: http://www.infoteca.cnptia.embrapa.br/handle/CNPDIA/9905 (accessed on 14 March 2011).
- Brun, A.; Chalot, M.; Botton, B.; Martin, F. Purification and Characterization of Glutamine Synthetase and NADP-Glutamate Dehydrogenase from the Ectomycorrhizal Fungus Laccaria laccata. Plant. Physiol. 1992, 99, 938–944. [Google Scholar] [CrossRef]
- Breuninger, M.; Trujillo, C.G.; Serrano, E.; Fischer, R.; Requena, N. Different nitrogen sources modulate activity but not expression of glutamine synthetase in arbuscular mycorrhizal fungi. Fungal Genet. Biol. 2004, 41, 542–552. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Scopes, R.K. Protein Purification: Principles and Practice, 3rd ed; Springer Verlag: New York, NY, USA, 1994; p. 380. [Google Scholar]
- Vasconcelos, E.A.R.; Nogueira, F.C.S.; Abreu, E.F.M.; Gonçalves, E.F.; Souza, P.A.S.; Campos, F.A.P. Protein extraction from cowpea tissues for 2D electrophoresis and MS analysis. Chromatographia 2005, 62, 447–450. [Google Scholar] [CrossRef]
- Cordeiro, R.A.; Brilhante, R.S.N.; Rocha, M.F.G.; Bandeira, S.P.; Fechine, M.A.B.; Camargo, Z.P.; Sidrim, J.J.C. Twelve years of coccidioidomycosis in Ceará State, Northeast Brazil: Epidemiologic and diagnostic aspects. Diagn. Microbiol. Infect. Dis. 2010, 66, 65–72. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the bacteriophage T4. Nature 1970, 227, 679–685. [Google Scholar]
- Nagao, A.T.; Martinez, C.C.; Vieira, V.S.; Takano, O.A.; Costa-Carvalho, B.T.; Carneiro-Sampaio, M.M.S. Carneiro-Sampaio, M.M.S. Placental transfer of IgG and IgG subclass antibodies anti-purified Escherichia coli LPS O16, O6 and O111. Scand. J. Immunol. Suppl. 1998, 47, 609–614. [Google Scholar] [CrossRef]
- Silva, F.D.A.; Vasconcelos, I.M.; Lobo, M.D.P.; Castro, P.G.; Magalhães, V.M.; Freitas, C.D.T.; Carlini, C.R.R.S.; Pinto, P.M.; Beltramini, L.M.; Filho, J.H.A.; et al. Biochemical, physicochemical and molecular characterization of a genuine 2-cys-peroxiredoxin purified from cowpea [Vigna unguiculata (L.) Walpers] leaves. Biochem. Biophys. Acta 2012, 1820, 1128–1140. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Filho, R.E.M.; Bandeira, S.P.; Brillhante, R.S.N.; Rocha, M.F.G.; Vasconcelos, I.M.; Pereira, M.L.; Castelo-Branco, D.d.S.C.M.; Rocha, F.A.C.; Camargo, Z.P.d.; Ramos, M.V.; et al. Biochemical Characterization of an In-House Coccidioides Antigen: Perspectives for the Immunodiagnosis of Coccidioidomycosis. Molecules 2012, 17, 7854-7863. https://doi.org/10.3390/molecules17077854
Filho REM, Bandeira SP, Brillhante RSN, Rocha MFG, Vasconcelos IM, Pereira ML, Castelo-Branco DdSCM, Rocha FAC, Camargo ZPd, Ramos MV, et al. Biochemical Characterization of an In-House Coccidioides Antigen: Perspectives for the Immunodiagnosis of Coccidioidomycosis. Molecules. 2012; 17(7):7854-7863. https://doi.org/10.3390/molecules17077854
Chicago/Turabian StyleFilho, Renato Evando Moreira, Silviane Praciano Bandeira, Raimunda Sâmia Nogueira Brillhante, Marcos Fábio Gadelha Rocha, Ilka Maria Vasconcelos, Mirella Leite Pereira, Débora de Souza Collares Maia Castelo-Branco, Francisco Airton Castro Rocha, Zoilo Pires de Camargo, Marcio Viana Ramos, and et al. 2012. "Biochemical Characterization of an In-House Coccidioides Antigen: Perspectives for the Immunodiagnosis of Coccidioidomycosis" Molecules 17, no. 7: 7854-7863. https://doi.org/10.3390/molecules17077854
APA StyleFilho, R. E. M., Bandeira, S. P., Brillhante, R. S. N., Rocha, M. F. G., Vasconcelos, I. M., Pereira, M. L., Castelo-Branco, D. d. S. C. M., Rocha, F. A. C., Camargo, Z. P. d., Ramos, M. V., Cordeiro, R. d. A., & Sidrim, J. J. C. (2012). Biochemical Characterization of an In-House Coccidioides Antigen: Perspectives for the Immunodiagnosis of Coccidioidomycosis. Molecules, 17(7), 7854-7863. https://doi.org/10.3390/molecules17077854