Observation of 1,3-Diketones Formation in the Reaction of Bulky Acyl Chlorides with Methyllithium
Abstract
:1. Introduction
2. Results and Discussion
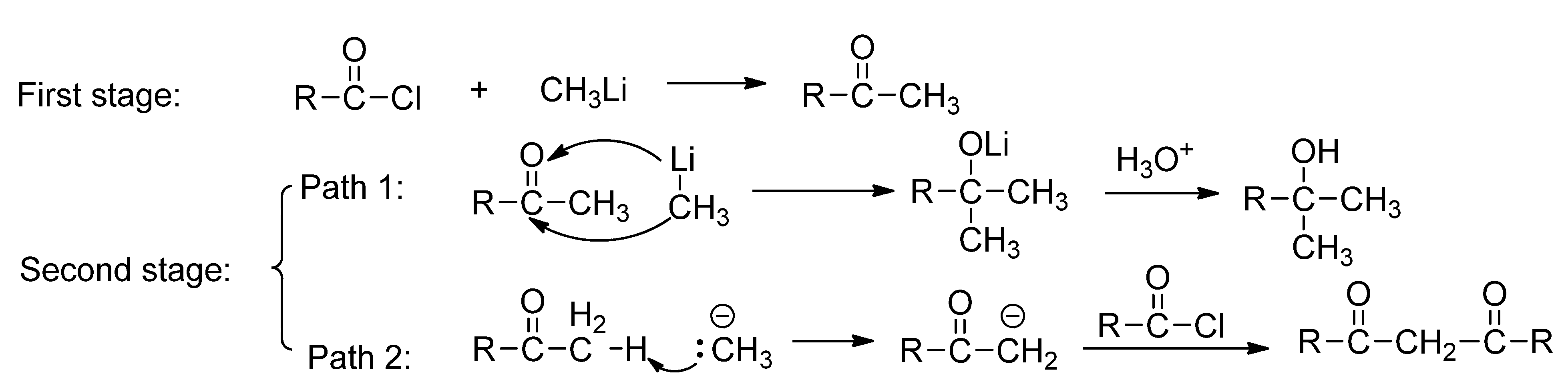
2.1. The Reaction of Menthylformyl Chloride with Methyllithium
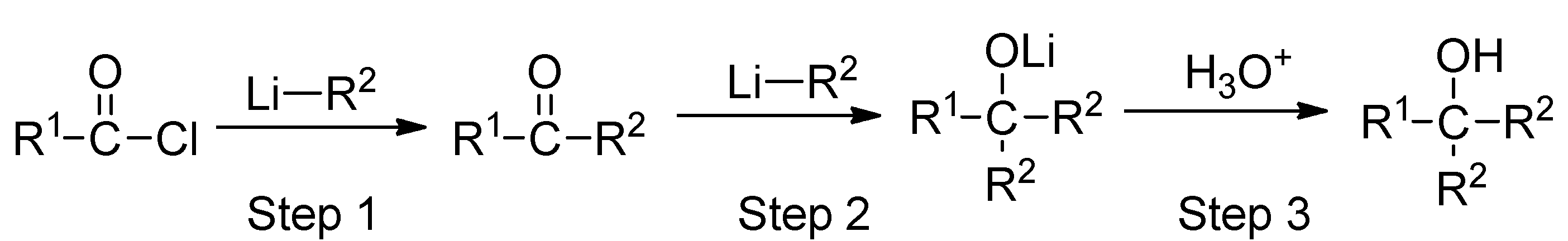
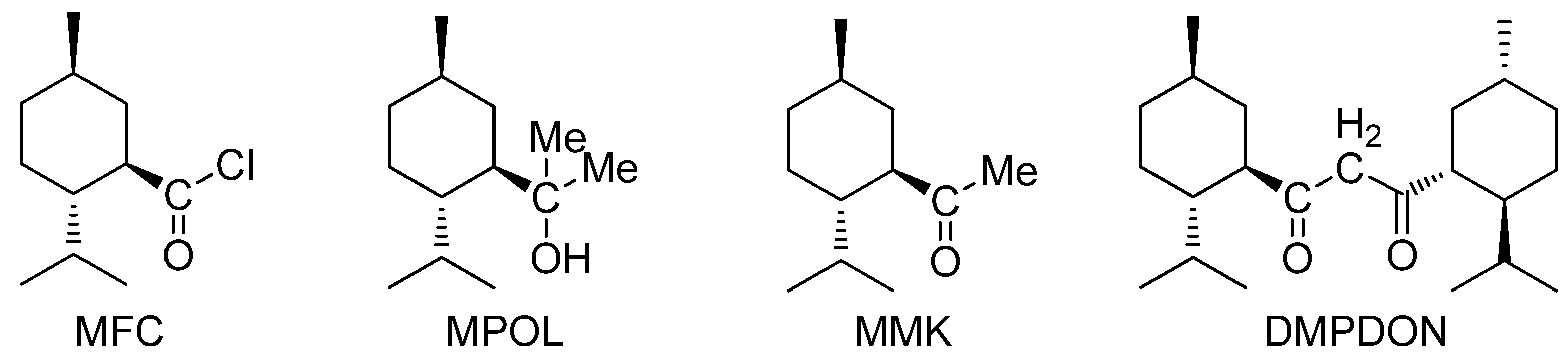
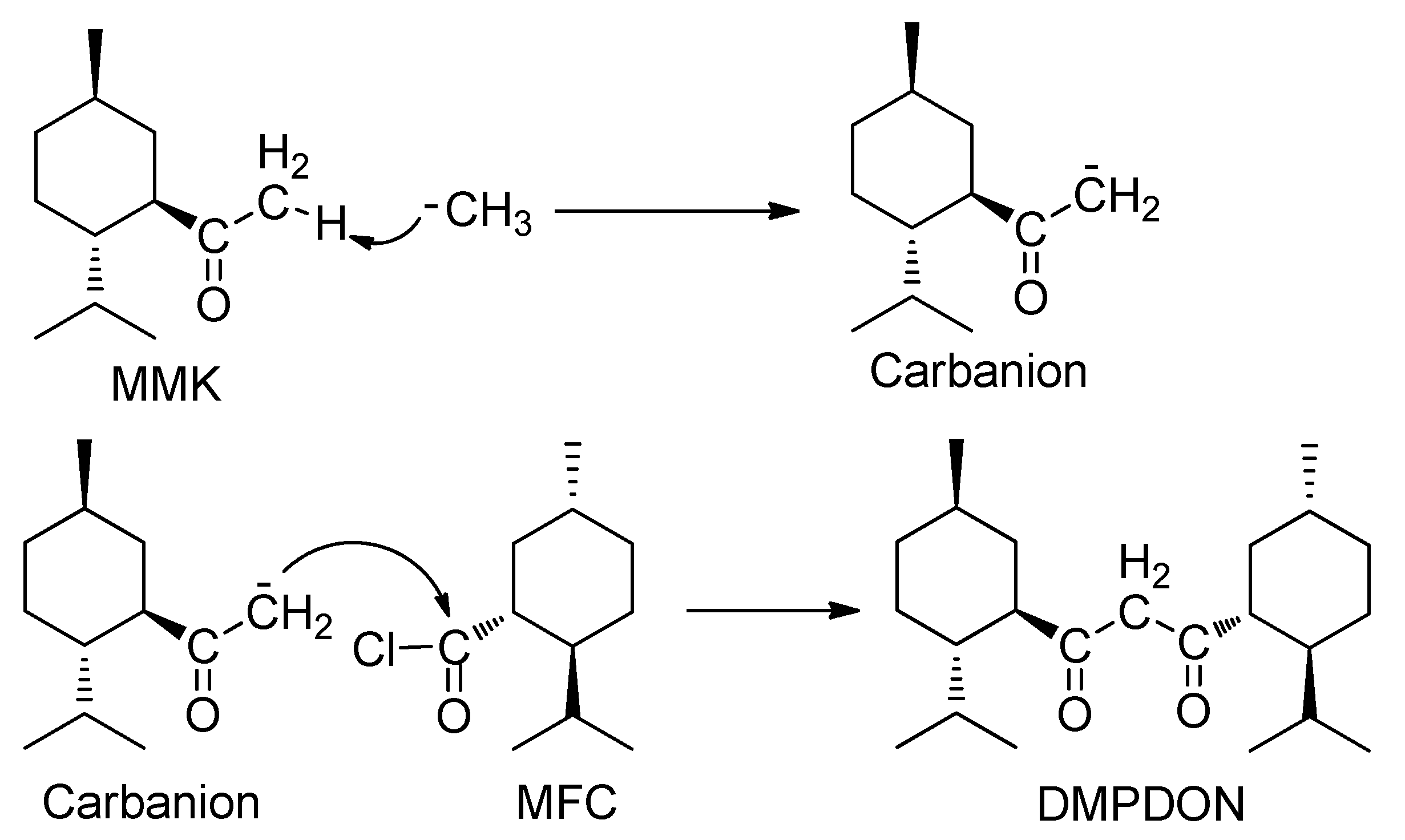
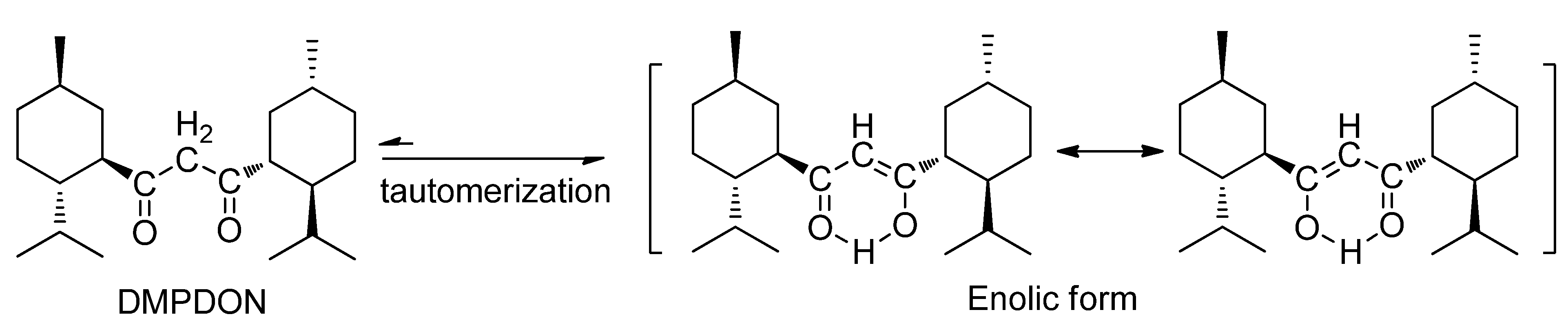
2.2. The Effect of the Reaction Condition on the Yield of 1,3-Dimenthylpropane-1,3-dione
| Run | Solvent b | MFC/MeLi c | Temperature (°C) | Yield (%) d |
|---|---|---|---|---|
| 1 | Toluene | 1/1.1 | −78 | 20.7 |
| 2 | Toluene | 1/1.1 | −5 | 61.3 |
| 3 | Toluene | 1/1.1 | 0 | 67.1 |
| 4 | Toluene | 1/1.1 | 25 | 40.5 |
| 5 | Toluene | 1/1.1 | 50 | 22.6 |
| 6 | Toluene | 1/1 | 0 | 65.2 |
| 7 | Toluene | 1/2.1 | 0 | 67.0 |
| 8 | Toluene | 1/3.1 | 0 | 67.1 |
| 9 e | Toluene | 1/1.1 | 0 | 57.4 |
| 10 | Tetrahydrofuran | 1/1.1 | 0 | 62.5 |
| 11 | Diethyl ether | 1/1.1 | 0 | 60.2 |
2.3. The Effect of the Bulk of Group Linking to Carbonyl of Acyl Chloride on the Reaction
| Run | R | Yield/% b | ||
|---|---|---|---|---|
| A | B | C | ||
| 1 |  | - | 67.0 | - |
| 2 |  | - | 34.0 | 36.1 |
| 3 c |  | 7.9 | 49.9 | 21.6 |
| 4 |  | - | 10.2 | 84.1 |
| 5 d |  | - | - | 91.1 |
| 6 |  | 69.4 | 12.2 | - |
3. Experimental
3.1. Instruments and Materials
3.2. The Typical Reaction Process
3.3. Chromatographic Eluents and Characterization of the Products
4. Conclusions
Acknowledgments
References and Notes
- Dieter, R.K. Reaction of acyl chlorides with organometallic reagents: A banquet table of metals for ketone synthesis. Tetrahedron 1999, 55, 4177–4236. [Google Scholar] [CrossRef]
- Posner, G.H.; Whitten, C.E.; McFarland, P.E. Organocopper chemistry. Halo-, cyano-, and carbonyl-substituted ketones from the corresponding acyl chlorides and organocopper reagents. J. Am. Chem. Soc. 1972, 94, 5106–5108. [Google Scholar] [CrossRef]
- Kel’in, A.V. Recent advances in the synthesis of 1,3-diketones. Curr. Org. Chem. 2003, 7, 1691–1711. [Google Scholar] [CrossRef]
- Kel’in, A.V.; Maioli, A. Recent advances in the chemistry of 1,3-diketones: Structural modifications and synthetic applications. Curr. Org. Chem. 2003, 7, 1855–1886. [Google Scholar] [CrossRef]
- Lim, D.; Fang, F.; Zhou, G.; Coltart, D.M. Direct carbon-carbon bond formation via soft enolization: A facile and efficient synthesis of 1,3-diketones. Org. Lett. 2007, 9, 4139–4142. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Smith, S.M.; Henson, S.; Kerrigan, N.J. Synthesis of 1,3-diketones through ring-opening of Ketoketene dimer β-lactones. Tetrahedron Lett. 2009, 50, 6919–6922. [Google Scholar] [CrossRef]
- Wu, S.; Yang, N.; Yang, L.; Cao, J.; Liu, J. A novel type of optically active helical polymers: Synthesis and characterization of poly(α,β-unsaturated ketone). J. Polym. Sci. A1 2010, 48, 1441–1448. [Google Scholar] [CrossRef]
- Jones, M.W.; Adlington, R.M.; Baldwin, J.E. New mononuclear Pd(II) complexes of sterically hindered bispyrazolylmethanes. Inorganica Chim. Acta 2010, 363, 1097–1101. [Google Scholar] [CrossRef]
- Zoidis, G.; Fytas, C.; Papanastasiou, I. Heterocyclic rimantadine analogues with antiviral activity. Bioorg. Med. Chem. 2006, 14, 3341–3348. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, P.J.; Yang, Y. High-yield synthesis of 1,3-dimesityl-propane-1,3-dione: Isolation of its aluminum complex as a stable intermediate. Synth. Commun. 2008, 38, 2349–2356. [Google Scholar] [CrossRef]
- Chapman, C.J.; Frost, C.G.; Hartley, J.P.; Whittle, A.J. Efficient aromatic and heteroatom acylations using catalytic indium complexes with lithium perchlorate. Tetrahedron Lett. 2001, 42, 773–775. [Google Scholar]
- Timberlake, J.W.; Pan, D.W.; Murray, J. Ortho methyl group effects in cumyl systems. J. Org. Chem. 1995, 60, 5295–5298. [Google Scholar] [CrossRef]
- Nandurkar, N.S.; Bhanushali, M.J.; Patil, D.S. Synthesis of sterically hindered 1,3-diketones. Synth. Commun. 2007, 37, 4111–4115. [Google Scholar] [CrossRef]
- Lempers, H.E.B.; Sheldon, R.A. Molybdenum-catalyzed epoxidations of oct-1-ene and cyclohexene with organic hydroperoxides: Steric effects of the alkyl substituents of the hydroperoxide on the reaction rate. Rec. Trav. Chim. Pays-Bas 1996, 115, 542–546. [Google Scholar]
- Matsumoto, T.; Takeda, Y.; Iwata, E.; Sakamoto, M.; Ishida, T. Lipase-catalyzed hydrolysis of some racemic 1-acetoxy-2-arylpropanes. Chem. Pharm. Bull. 1994, 42, 1191–1197. [Google Scholar] [CrossRef]
- Hog, D.T.; Oestreich, M. B(C6F5)3-catalyzed reduction of ketones and imines using silicon-stereogenic silanes: Stereoinduction by single-point binding. Eur. J. Org. Chem. 2009, 29, 5047–5056. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Yang, N.; Yang, L. Observation of 1,3-Diketones Formation in the Reaction of Bulky Acyl Chlorides with Methyllithium. Molecules 2012, 17, 6415-6423. https://doi.org/10.3390/molecules17066415
Zhang J, Yang N, Yang L. Observation of 1,3-Diketones Formation in the Reaction of Bulky Acyl Chlorides with Methyllithium. Molecules. 2012; 17(6):6415-6423. https://doi.org/10.3390/molecules17066415
Chicago/Turabian StyleZhang, Jian, Nianfa Yang, and Liwen Yang. 2012. "Observation of 1,3-Diketones Formation in the Reaction of Bulky Acyl Chlorides with Methyllithium" Molecules 17, no. 6: 6415-6423. https://doi.org/10.3390/molecules17066415
APA StyleZhang, J., Yang, N., & Yang, L. (2012). Observation of 1,3-Diketones Formation in the Reaction of Bulky Acyl Chlorides with Methyllithium. Molecules, 17(6), 6415-6423. https://doi.org/10.3390/molecules17066415





