Glycosylation of Vanillin and 8-Nordihydrocapsaicin by Cultured Eucalyptus perriniana Cells
Abstract
:1. Introduction
2. Results and Discussion
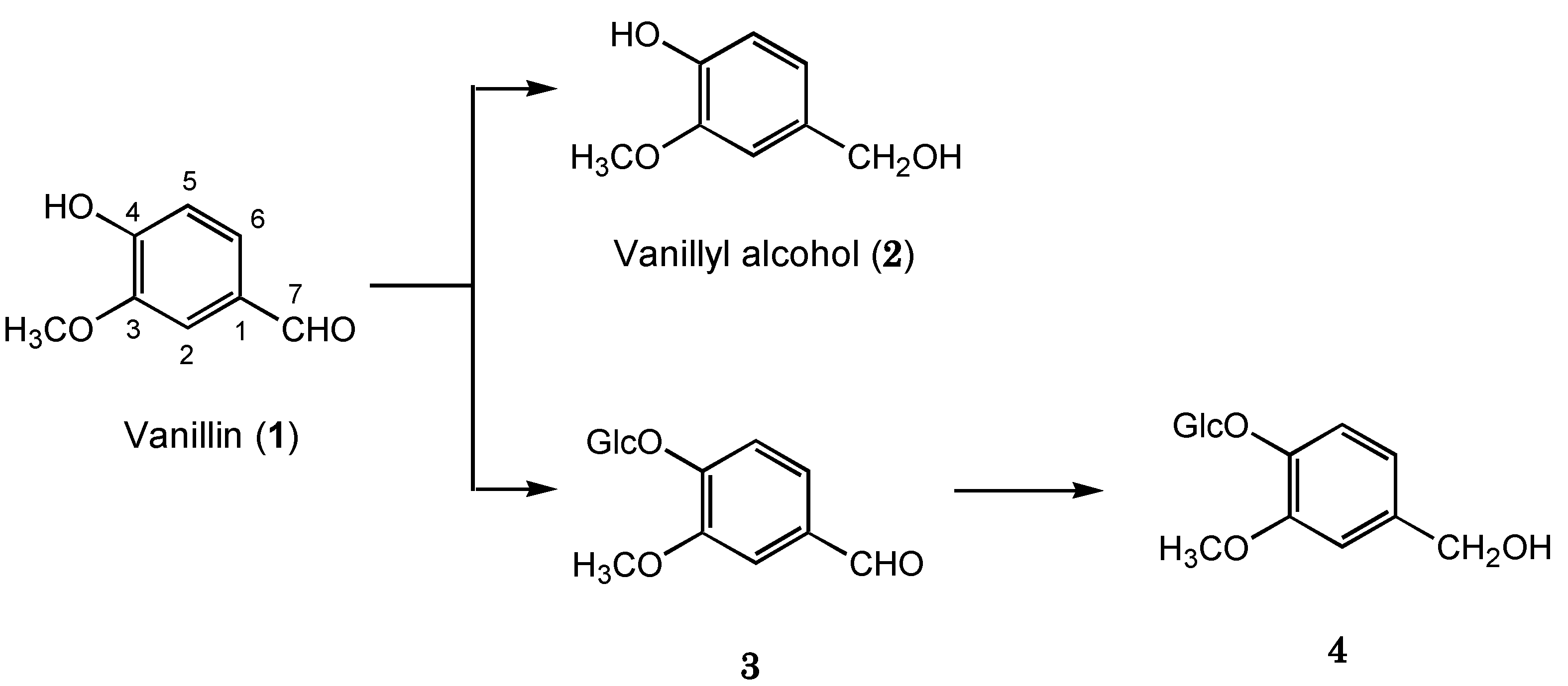
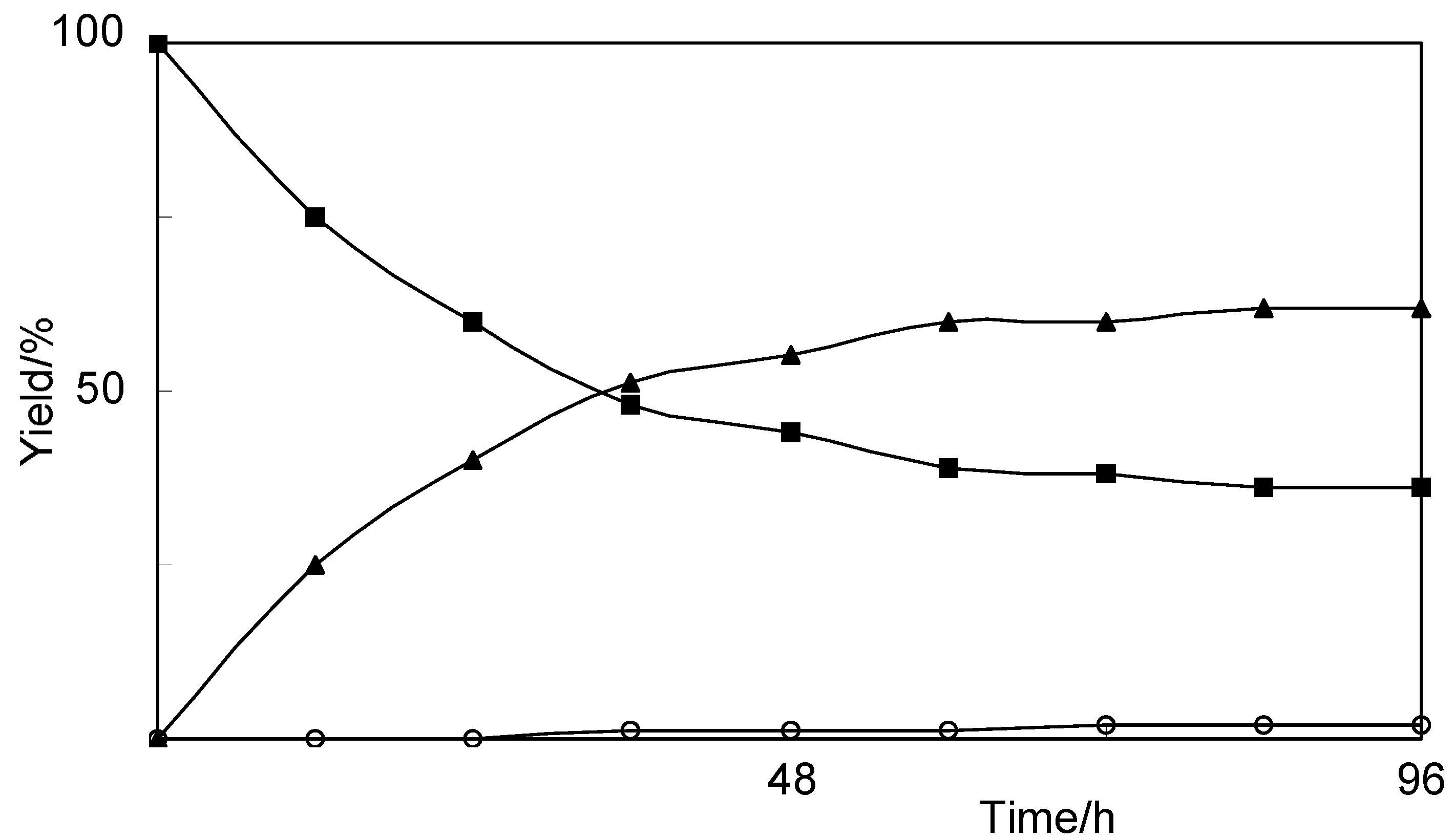
2.1. Biotransformation of Vanillin
| Compound | 3 | 4 | 6 | 7 | |
|---|---|---|---|---|---|
| Aglycone | 1 | 130.2 | 136.0 | 134.8 | 135.1 |
| 2 | 110.1 | 110.7 | 113.0 | 113.1 | |
| 3 | 151.4 | 148.4 | 150.5 | 150.8 | |
| 4 | 148.9 | 144.9 | 146.8 | 146.7 | |
| 5 | 114.2 | 114.8 | 117.9 | 118.3 | |
| 6 | 125.1 | 118.2 | 121.1 | 121.4 | |
| 7 | 191.2 | 62.5 | 43.6 | 43.8 | |
| 8 | 175.7 | 176.0 | |||
| 9 | 37.0 | 37.1 | |||
| 10 | 27.0 | 27.1 | |||
| 11 | 30.3 | 30.3 | |||
| 12 | 30.3 | 30.3 | |||
| 13 | 30.3 | 30.3 | |||
| 14 | 32.9 | 32.9 | |||
| 15 | 23.6 | 23.7 | |||
| 16 | 14.4 | 14.4 | |||
| OCH3 | 55.4 | 55.3 | 56.6 | 56.7 | |
| Glc | 1' | 99.1 | 99.9 | 102.7 | 102.5 |
| 2' | 72.8 | 73.0 | 74.7 | 75.1 | |
| 3' | 76.9 | 76.7 | 77.9 | 77.8 | |
| 4' | 69.3 | 69.4 | 71.2 | 71.5 | |
| 5' | 76.6 | 76.6 | 77.6 | 77.6 | |
| 6' | 60.3 | 60.4 | 62.4 | 69.4 | |
| 1'' | 104.3 | ||||
| 2'' | 74.8 | ||||
| 3'' | 77.6 | ||||
| 4'' | 71.3 | ||||
| 5'' | 77.8 | ||||
| 6'' | 62.6 |

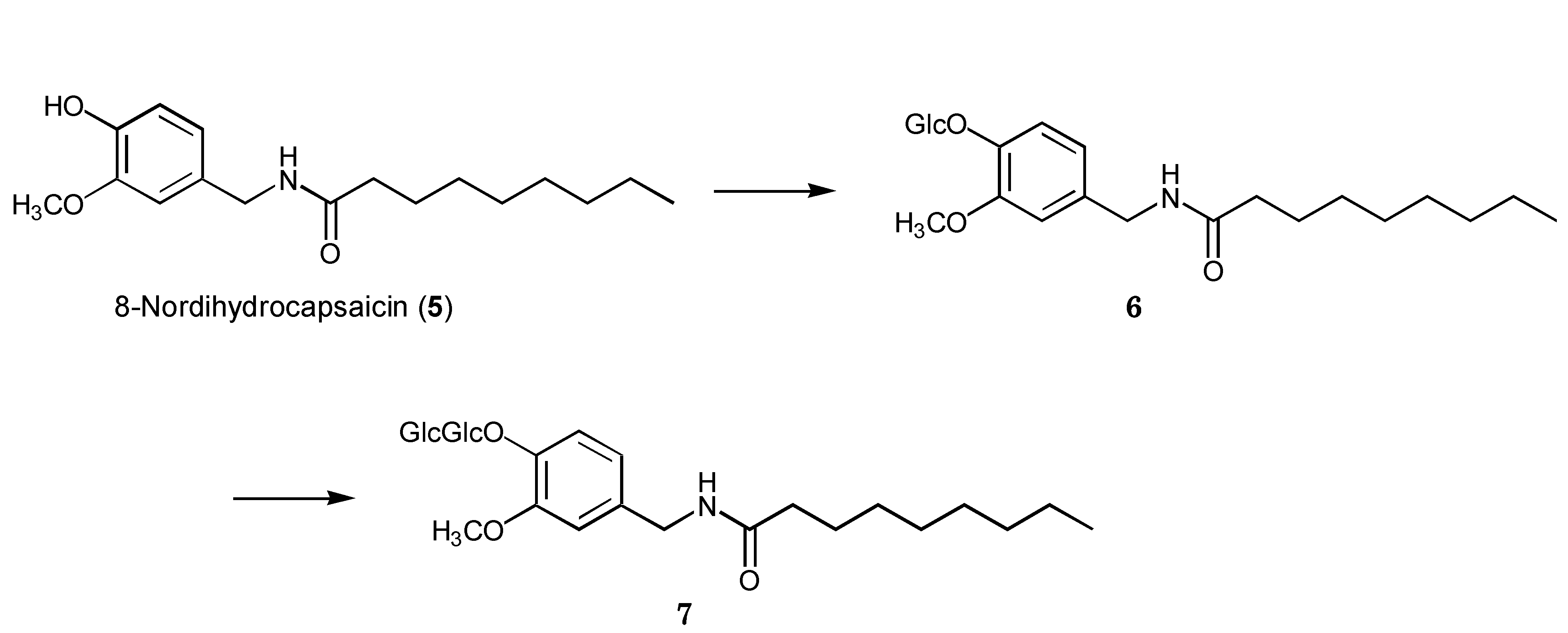
2.2. Biotransformation of 8-Nordihydrocapsaicin
3. Experimental
3.1. Analysis of the Products
3.2. Cell Line and Culture Conditions
3.3. Biotransformation and Purification of Products
4. Conclusions
Supplementary Materials
Conflict of Interest
References and Notes
- Ishihara, K.; Hamada, H.; Hirata, T.; Nakajima, N. Biotransformation using plant cultured cells. J. Mol. Catal. B: Enz. 2003, 23, 145–170. [Google Scholar] [CrossRef]
- Hamada, H.; Kondo, Y.; Ishihara, K.; Nakajima, N.; Hamada, H.; Kurihara, R.; Hirata, T. Stereoselective biotransformation of limonene and limonene oxide by cyanobacterium. J. Biosci. Bioeng. 2003, 96, 581–584. [Google Scholar] [CrossRef]
- Takenaka, S.; Mulyono; Sasano, Y.; Takahashi, Y.; Murakami, S.; Aoki, K. Microbial transformation of aniline derivatives: Regioselective biotransformation and detoxification of 2-phenylenediamine by Bacillus cereus strain PDa-1. J. Biosci. Bioeng. 2006, 102, 21–27. [Google Scholar] [CrossRef]
- Mulyono; Takenaka, S.; Sasano, Y.; Murakami, S.; Aoki, K. Bacillus cereus strain 10-L-2 produces two arylamine N-acetyltransferases that transform 4-phenylenediamine into 4-aminoacetanilide. J. Biosci. Bioeng. 2007, 103, 147–154. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Bai, H.; Gong, J.; Wang, Y.; Li, B.; Li, J. Biotransformation of β-amyrin acetate by Rhodobacter sphaeroides. J. Biosci. Bioeng. 2008, 105, 558–561. [Google Scholar] [CrossRef]
- Furuya, T.; Ushiyama, M.; Ashida, Y.; Yoshikawa, T. Biotransformation of 2-phenylpropionic acid in root culture of Panax ginseng. Phytochemistry 1989, 28, 483–487. [Google Scholar]
- Kamel, S.; Brazier, M.; Desmet, G.; Fliniaux, M.-A.; Jacquin-Dubreuil, A. Glucosylation of butyric acid by cell suspension culture of Nicotiana plumbaginifolia. Phytochemistry 1992, 31, 1581–1583. [Google Scholar]
- Lewinson, E.; Berman, E.; Mazur, Y.; Gressel, J. Glucosylation of exogenous flavanones by grapefruit (Citrus paradisi) cell cultures. Phytochemistry 1996, 25, 2531–2535. [Google Scholar]
- Shimoda, K.; Kondo, Y.; Abe, K.; Hamada, H.; Hamada, H. Formation of water-soluble vitamin derivatives from lipophilic vitamins by cultured plant cells. Tetrahedron Lett. 2006, 47, 2695–2698. [Google Scholar]
- Shimoda, K.; Kondo, Y.; Nishida, T.; Hamada, H.; Nakajima, N.; Hamada, H. Biotransformation of thymol, carvacrol, and eugenol by cultured cells of Eucalyptus perrinian. Phytochemistry 2006, 67, 2256–2261. [Google Scholar] [CrossRef]
- Shimoda, K.; Harada, T.; Hamada, H.; Nakajima, N.; Hamada, H. Biotransformation of raspberry ketone and zingerone by cultured cells of Phytolacca americana. Phytochemistry 2007, 68, 487–492. [Google Scholar]
- Shimoda, K.; Hamada, H.; Hamada, H. Glycosylation of hesperetin by plant cell cultures. Phytochemistry 2008, 69, 1135–1140. [Google Scholar]
- Shimoda, K.; Kondo, Y.; Akagi, M.; Abe, K.; Hamada, H.; Hamada, H. Glycosylation of tocopherols by cultured cells of Eucalyptus perriniana. Phytochemistry 2007, 68, 2678–2683. [Google Scholar] [CrossRef]
- Burri, J.; Graf, M.; Lambelet, P.; Loliger, J. Vanillin: more than a flavouring agent-a potent antioxidant. J. Sci. Food Agric. 1989, 48, 49–56. [Google Scholar] [CrossRef]
- Surh, Y.J.; Lee, S.S. Capsaicin, a double edged sword: toxicity, metabolism, and chemopreventative potential (Review). Life Sci. 1995, 56, 1845–1855. [Google Scholar] [CrossRef]
- Surh, Y.J.; Ahn, S.H.; Kim, K.C.; Park, J.B.; Sohn, Y.W.; Lee, S.S. Metabolism of capsaicinoids: evidence for aliphatic hydroxylation and its pharmacological implications. Life Sci. 1995, 56, PL305–PL311. [Google Scholar] [CrossRef]
- Surh, Y.J.; Lee, E.; Lee, J.M. Chemopreventive properties of some pungent ingredients present in red pepper and ginger. Mut. Res. 1998, 402, 259–267. [Google Scholar] [CrossRef]
- Park, K.K.; Chun, K.S.; Yook, J.I.; Surh, Y.J. Lack of tumor promoting activity of capsaicin, a principal pungent ingredient of red peppers, in mouse skin carcinogens. Anticancer Res. 1998, 18, 4201–4205. [Google Scholar]
- Ward, M.H.; Lopez-Carrillo, L. Dietary factors and the risk of gastric cancer in Mexico City. Am. J. Epid. 1999, 149, 925–932. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawada, T.; Yamamoto, M.; Iwai, K. Capsaicin, a pungent principle of hot red pepper, evokes catecholamine secretion from the adrenal medulla of anesthetized rats. Biochem. Biophys. Res. Commun. 1987, 142, 259–264. [Google Scholar] [CrossRef]
- Kometani, T.; Tanimoto, H.; Nishimura, T.; Okada, S. Glucosylation of vanillin by cultured plant cells. Biosci. Biotech. Biochem. 1993, 57, 1290–1293. [Google Scholar] [CrossRef]
- Yuana; Dignum, M.J.W.; Verpoorte, R. Glucosylation of exogenous vanillin by plant cell cultures. Plant Cell Tiss. Org. Cult. 2002, 69, 177–182. [Google Scholar] [CrossRef]
- Higashiguchi, F.; Nakamura, H.; Hayashi, H.; Kometani, T. Purification and structure determination of glucosides of capsaicin and dihydrocapsaicin from various capsicum fruits. J. Agric. Food Chem. 2006, 54, 5948–5953. [Google Scholar] [CrossRef]
- Hamada, H.; Fuchikami, Y.; Ikematsu, Y.; Hirata, T.; Williams, H.; Scott, A.I. Hydroxylation of piperitone by cell suspension cultures of Catharanthus roseus. Phytochemistry 1994, 37, 1037–1038. [Google Scholar]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef]
- Shimoda, K.; Kwon, S.; Utsuki, A.; Ohiwa, S.; Katsuragi, H.; Yonemoto, N.; Hamada, H.; Hamada, H. Glycosylation of capsaicin and 8-nordihydrocapsaicin by cultured cells of Catharanthus roseus. Phytochemistry 2007, 68, 1391–1396. [Google Scholar]
- Sultana, I.; Shimamoto, M.; Obata, R.; Nishiyama, S.; Sugai, T. An expeditious chemo-enzymatic synthesis of dihydronorcapsaisin β-D-glucopyranoside. Sci. Technol. Adv. Mater. 2006, 7, 197–201. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sato, D.; Eshita, Y.; Katsuragi, H.; Hamada, H.; Shimoda, K.; Kubota, N. Glycosylation of Vanillin and 8-Nordihydrocapsaicin by Cultured Eucalyptus perriniana Cells. Molecules 2012, 17, 5013-5020. https://doi.org/10.3390/molecules17055013
Sato D, Eshita Y, Katsuragi H, Hamada H, Shimoda K, Kubota N. Glycosylation of Vanillin and 8-Nordihydrocapsaicin by Cultured Eucalyptus perriniana Cells. Molecules. 2012; 17(5):5013-5020. https://doi.org/10.3390/molecules17055013
Chicago/Turabian StyleSato, Daisuke, Yuki Eshita, Hisashi Katsuragi, Hiroki Hamada, Kei Shimoda, and Naoji Kubota. 2012. "Glycosylation of Vanillin and 8-Nordihydrocapsaicin by Cultured Eucalyptus perriniana Cells" Molecules 17, no. 5: 5013-5020. https://doi.org/10.3390/molecules17055013




