Synthesis and Characterization of ZnO Nanowires by Thermal Oxidation of Zn Thin Films at Various Temperatures
Abstract
:1. Introduction
2. Results and Discussion
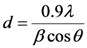
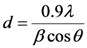
 ,
,  and
and  are the X-ray wavelength (0.154 nm), Bragg diffraction angle and FWHM, respectively. The mean grain sizes of the samples were 60 nm, 63 nm, 70 nm for the samples oxidized at 500 °C, 550 °C and 600 °C, respectively, which indicates that the particle size increases with the increasing oxidation temperature. However, by further increasing the temperature to 650 °C, this value decreased, as the mean grain size for the sample oxidized at 650 °C was 60 nm. We think that the results are probably due to the deformation of the glass substrates at 650 °C.
are the X-ray wavelength (0.154 nm), Bragg diffraction angle and FWHM, respectively. The mean grain sizes of the samples were 60 nm, 63 nm, 70 nm for the samples oxidized at 500 °C, 550 °C and 600 °C, respectively, which indicates that the particle size increases with the increasing oxidation temperature. However, by further increasing the temperature to 650 °C, this value decreased, as the mean grain size for the sample oxidized at 650 °C was 60 nm. We think that the results are probably due to the deformation of the glass substrates at 650 °C.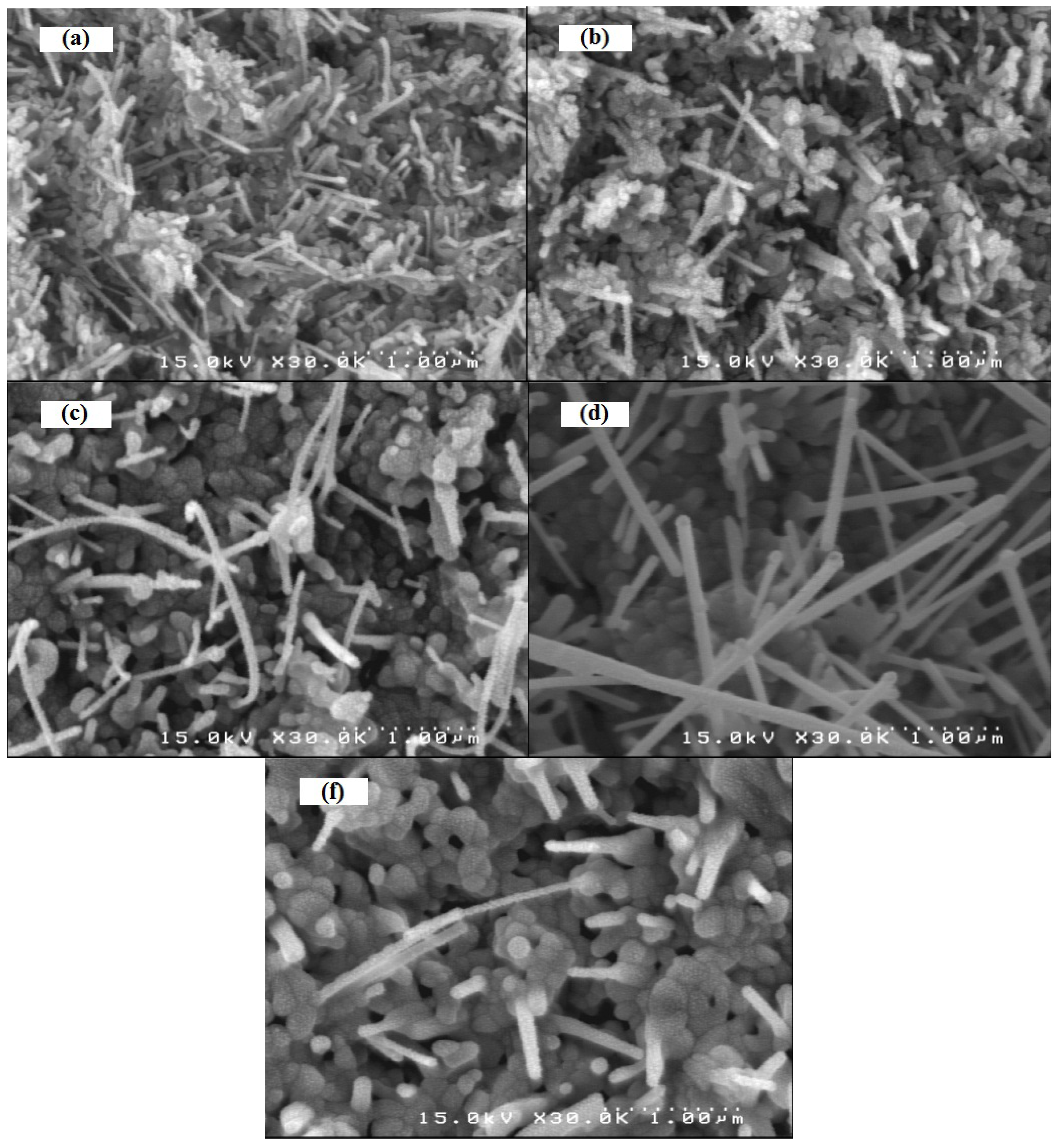
 2ZnO. These ZnO nuclei individually further grow in the upper direction in the form of nanowires. From the SEM image (Figure 3), we can observe that the individual Zn grains are oxidized at 600 °C, during a short time of oxidation (~30 min) that shows nanowires growing from individual Zn grains. This growth mechanism ensures that the nanowires are grown with low defects.
2ZnO. These ZnO nuclei individually further grow in the upper direction in the form of nanowires. From the SEM image (Figure 3), we can observe that the individual Zn grains are oxidized at 600 °C, during a short time of oxidation (~30 min) that shows nanowires growing from individual Zn grains. This growth mechanism ensures that the nanowires are grown with low defects.

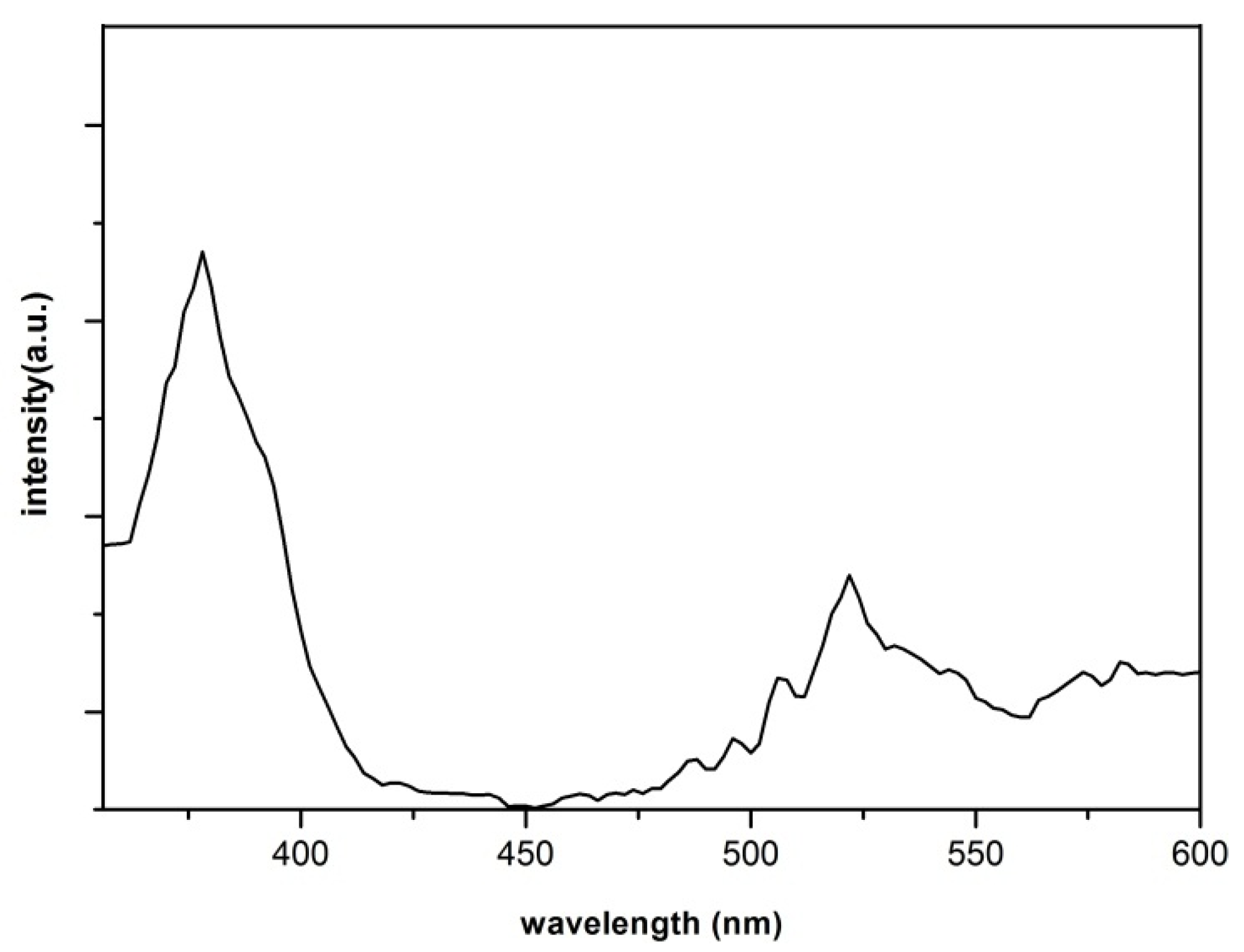
3. Experimental
4. Conclusions
Acknowledgements
References and Notes
- Duan, X.F.; Huang, Y.; Cui, Y.; Wang, J.F.; Lieber, C.M. Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature 2001, 409, 66–69. [Google Scholar] [CrossRef]
- Kind, H.; Yan, H.; Law, M.; Messer, B.; Yang, P. Nanowire ultraviolet photodetectors and optical switches. Adv. Mater. 2002, 14, 158–160. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, X.; Cui, Y.; Laubon, L.J.; Kim, K.H.; Lieber, C.M. Logic gates and computation from assembled nanowire building blocks. Science 2001, 294, 1313–1317. [Google Scholar]
- Wang, X.D.; Zhou, J.; Song, J.H.; Liu, J.; Xu, N.S.; Wang, Z.L. Piezoelectric field effect transistor and nanoforce sensor based on a single ZnO nanowire. Nano Lett. 2006, 6, 2768–2772. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P.D. Nanowire dye-sensitized solar cell. Nat. Mater 2005, 4, 455–459. [Google Scholar] [CrossRef]
- Lim, J.H.; Kang, C.K.; Kim, K.K.; Park, I.K.; Hwang, D.K.; Park, S.J. UV electroluminescence emission from ZnO eight-Emitting diodes grown by high temperature radiofrequency sputtering. Adv. Mater. 2006, 18, 2720–2724. [Google Scholar] [CrossRef]
- Ikegami, K.; Yoshiyama, T.; Maejima, K.; Shibata, H.; Tampo, H.; Niki, S. Optical dielectric constant inhomogeneity along the growth axis in ZnO-based transparent electrodes deposited on glass substrates. J. Appl. Phys. 2009, 105, 093713–093719. [Google Scholar]
- Wan, Q.; Lin, C.L.; Yu, X.B.; Wang, T.H. Room-temperature hydrogen storage characteristics of ZnO nanowires. Appl. Phys. Lett. 2004, 84, 124–126. [Google Scholar]
- Zhang, N.; Yi, R.; Shi, R.R.; Gao, G.H.; Chen, G.; Liu, X.H. Novel rose-like ZnO nanoflowers synthesized by chemical vapor deposition. Mater. Lett. 2009, 63, 496–499. [Google Scholar]
- Varanasi, C.V.; Leedy, K.D.; Tomich, D.H.; Subramanyam, G.; Look, D.C. Improved photoluminescence of vertically aligned ZnO nanorods grown on BaSrTiO3 by pulsed laser deposition. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Ham, H.; Shen, G.; Cho, J.H.; Lee, T.J.; Seo, S.H.; Lee, C.J. Vertically aligned ZnO nanowires produced by a catalyst-free thermal evaporation method and their field emission properties. Chem. Phys. Lett. 2005, 404, 69–73. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, S.C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Cho, S.; Ma, J.; Kim, Y.; Sun, Y.; Wong, G.K.L.; Ketterson, J.B. Photoluminescence and ultraviolet lasing of polycrystalline ZnO thin films prepared by the oxidation of the metallic Zn. Appl. Phys. Lett. 1999, 75, 2761–2763. [Google Scholar]
- Zhao, J.; Hu, L.Z.; Wang, Z.Y.; Zhao, Y.; Liang, X.P.; Wang, M.T. High-quality ZnO thin films prepared by low temperature oxidation of metallic Zn. Appl. Surf. Sci. 2004, 229, 311–315. [Google Scholar] [CrossRef]
- Wang, Y.G.; Lau, S.P.; Lee, H.W.; Yu, S.F.; Tay, B.K.; Zhang, X.H. Photoluminescence study of ZnO films prepared by thermal oxidation of Zn metallic films in air. J. Appl. Phys. 2003, 94, 354–358. [Google Scholar] [CrossRef]
- Dang, H.Y.; Wang, J.; Fan, S.S. The synthesis of metal oxide nanowires by directly heating metal samples in appropriate oxygen atmospheres. Nanotechnology 2003, 14, 738–741. [Google Scholar] [CrossRef]
- Kim, T.W.; Kawazoe, T.; Yamazaki, S.; Ohtsu, M.; Sekiguchi, T. Low-temperature orientation-selective growth and ultraviolet emission of single-crystal ZnO nanowires. Appl. Phys. Lett. 2004, 84, 3358–3360. [Google Scholar]
- Srivatsa, K.M.K; Chhikara, D; Kumar, S. Synthesis of aligned ZnO nanorod array on silicon and sapphire substrate bye thermal evaporation technique. J. Mater. Sci. Technol. 2011, 27, 701–706. [Google Scholar] [CrossRef]
- Dai, L.; Chen, X.L.; Wang, W.J.; Zhou, T.; Hu, B.Q. Growth and luminescence characterization of large-scale zinc oxide nanowires. J. Phys. Condens. Matter 2003, 15, 2221–2226. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison Wesley: Reading, MA, USA, 1978; p. 102. [Google Scholar]
- Umar, A.; Jeong, J.P.; Suh, E.K.; Hahn, Y.B. Synthesis of ZnO nanowires on steel alloy substrate by thermal evaporation: Growth mechanism and structural and optical properties. Korean J. Chem. Eng. 2006, 23, 860–865. [Google Scholar] [CrossRef]
- Geng, C.Y.; Jiang, Y.; Yao, Y.; Meng, X.M.; Zapien, J.A.; Lee, C.S.; Lifshitz, Y.; Lee, S.T. Well-aligned ZnO nanowire arrays fabricated on silicon substrates. Adv. Func. Mater. 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Wang, L.S.; Zhang, X.Z.; Zhao, S.Q.; Zhou, G.Y.; Zhou, Y.L.; Qi, J.J. Synthesis of well-aligned ZnO nanowires by simple physical vapor deposition on c-oriented ZnO thin films without catalysts or additives. Appl. Phys. Lett. 2005, 86, 024108–024110. [Google Scholar]
- Yang, P.; Lieber, C.M. Nanostructured high-temperature superconductors: Creation of strong-pinning columnar defects in nanorod/superconductor composites. J. Mater. Res. 1997, 12, 2981–2996. [Google Scholar] [CrossRef]
- Kar, S.; Pal, B.N.; Chaudhuri, S.; Chakravorty, D. One-dimensional ZnO nanostructure arrays: Synthesis and characterization. J. Phys. Chem. B 2006, 110, 4605–4611. [Google Scholar] [CrossRef]
- Kim, S.H.; Umar, A.; Hahn, Y.B. Growth and formation mechanism of sea urchin-like ZnO nanostructures on Si. Korean J. Chem. Eng. 2005, 22, 489–493. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407–409. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, K.H.; Chen, S.Y. Growth and characterization of Zn-ZnO core-shell polygon prismatic nanocrystal on Si. J. Cryst. Growth 2004, 269, 425–431. [Google Scholar] [CrossRef]
- Xu, L.F.; Guo, Y.; Liao, Q.; Zhang, J.P.; Xu, L.S. Morphological control of ZnO nanostructures by electrodeposition. J. Phys. Chem. B 2005, 109, 13519–13522. [Google Scholar]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar]
- Sample Availability: Samples of the compounds ZnO nanowires are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khanlary, M.R.; Vahedi, V.; Reyhani, A. Synthesis and Characterization of ZnO Nanowires by Thermal Oxidation of Zn Thin Films at Various Temperatures. Molecules 2012, 17, 5021-5029. https://doi.org/10.3390/molecules17055021
Khanlary MR, Vahedi V, Reyhani A. Synthesis and Characterization of ZnO Nanowires by Thermal Oxidation of Zn Thin Films at Various Temperatures. Molecules. 2012; 17(5):5021-5029. https://doi.org/10.3390/molecules17055021
Chicago/Turabian StyleKhanlary, Mohammad Reza, Vahid Vahedi, and Ali Reyhani. 2012. "Synthesis and Characterization of ZnO Nanowires by Thermal Oxidation of Zn Thin Films at Various Temperatures" Molecules 17, no. 5: 5021-5029. https://doi.org/10.3390/molecules17055021



