An Improved Synthesis of Pentacene: Rapid Access to a Benchmark Organic Semiconductor
Abstract
:1. Introduction
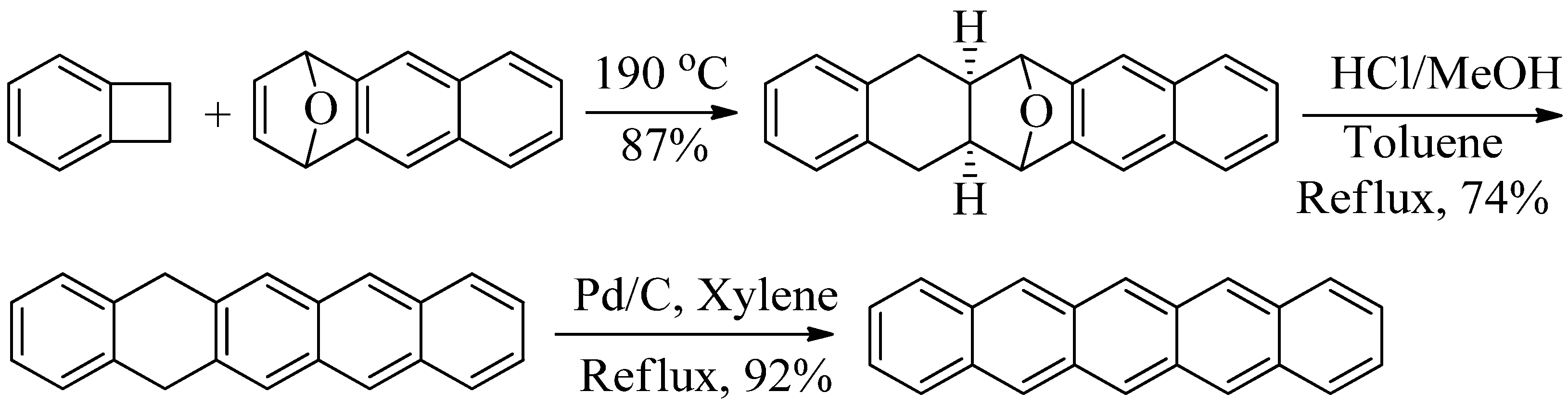
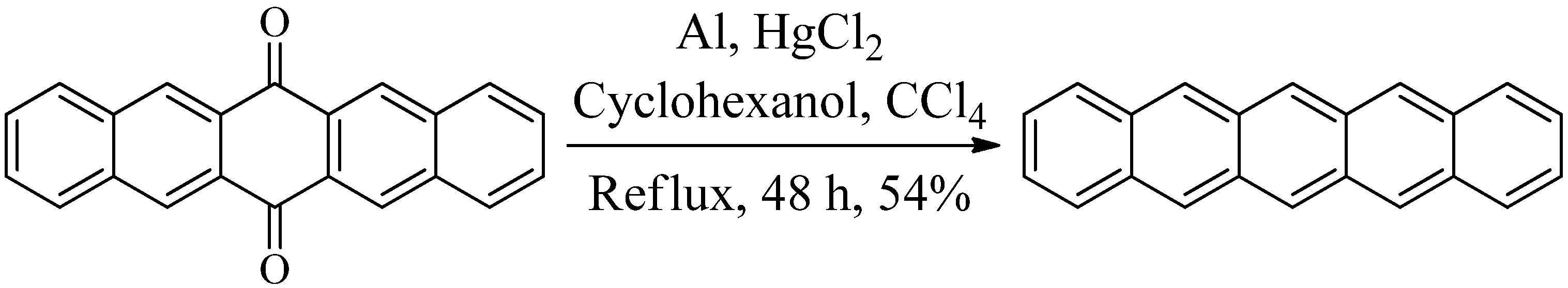


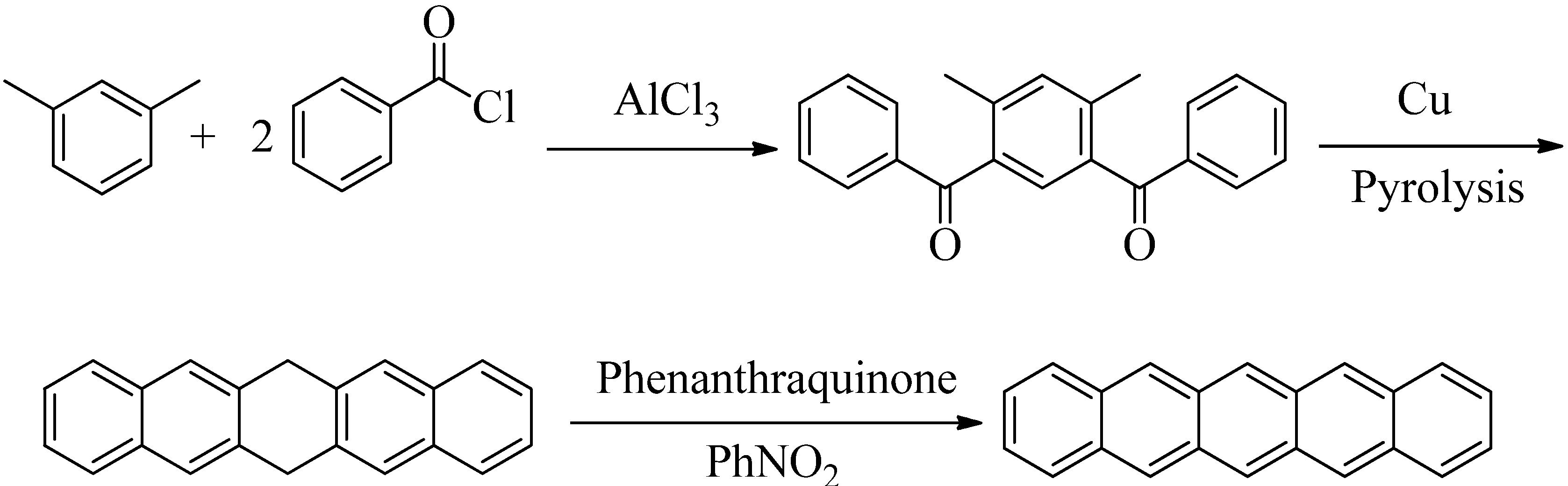
2. Results and Discussion
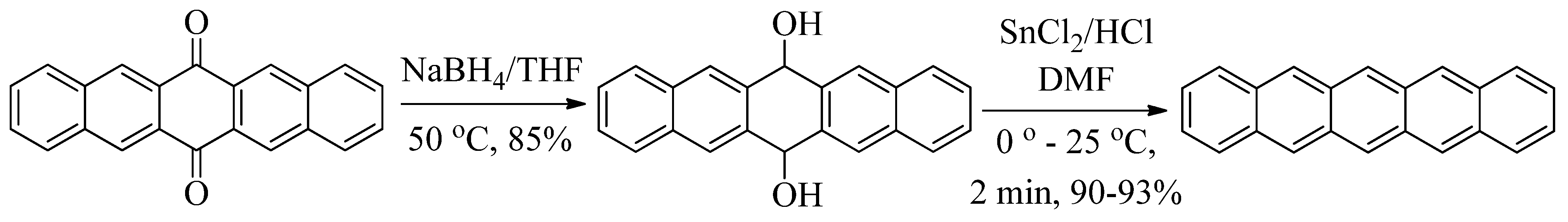

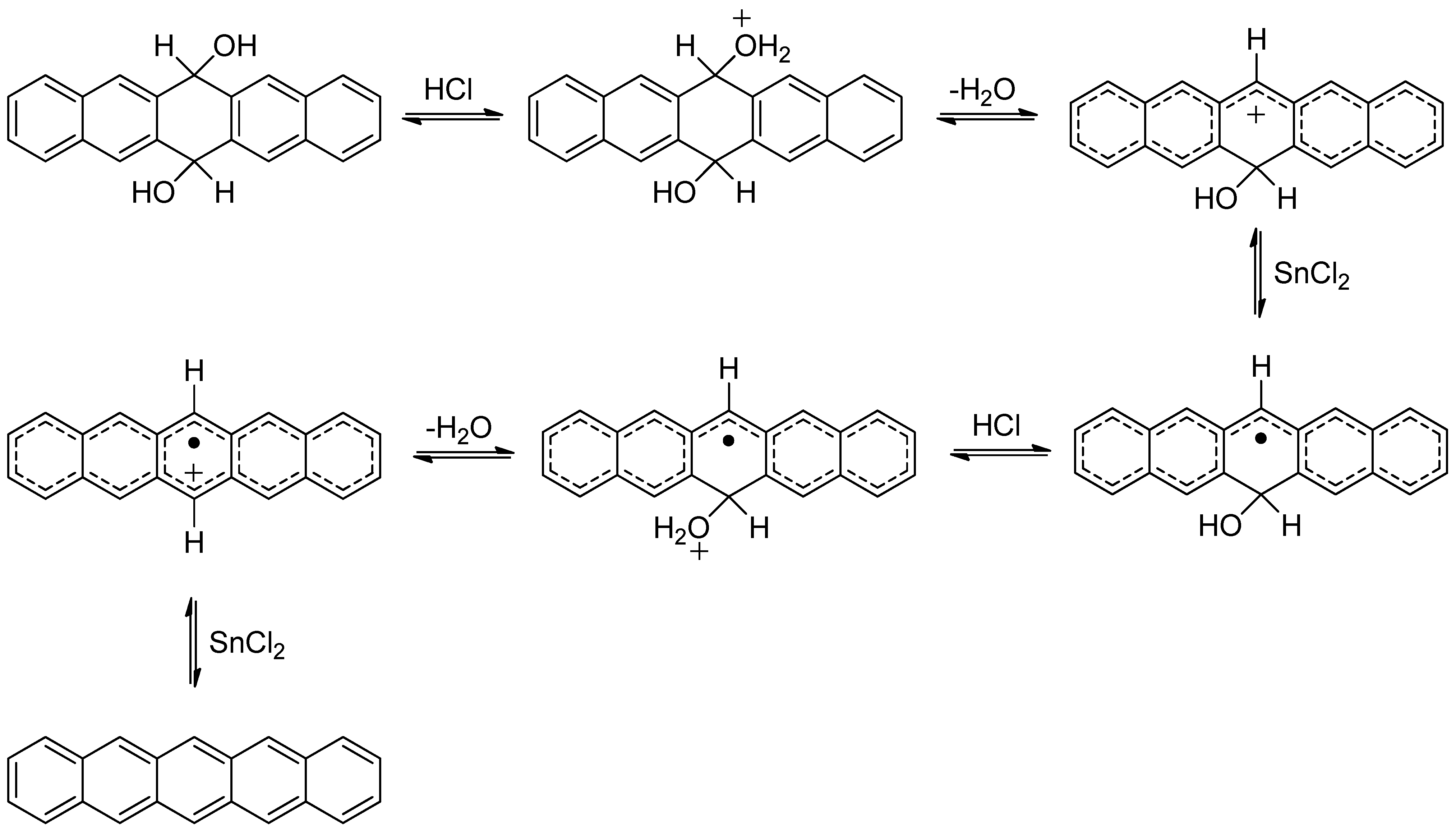
3. Experimental
3.1. General Experimental Methods
4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- Karl, N. Charge carrier transport in organic semiconductors. Synth. Met. 2003, 133, 649–657. [Google Scholar] [CrossRef]
- Yamashita, Y. Organic semiconductors for organic field-effect transistors. Sci. Technol. Adv. Mater. 2009, 10, 1–9. [Google Scholar] [CrossRef]
- Lin, Y.; Gundlach, D.J.; Nelson, S.F.; Jackson, T.N. Stacked pentacene layer organic thin-film transistors with improved characteristics. IEEE Electron Device Lett. 1997, 18, 606–608. [Google Scholar] [CrossRef]
- Dimitrakopoulos, C.D.; Malenfant, P.R.L. Organic thin film transistors for large area electronics. Adv. Mat. 2002, 14, 99–117. [Google Scholar] [CrossRef]
- Shi, S.; Ma, D. A pentacene-doped hole injection layer for organic light-emitting diodes. Semicond. Sci. Technol. 2005, 20, 1213–1216. [Google Scholar] [CrossRef]
- Rao, M.V.M.; Huang, T.; Su, Y.; Huang, Y. Fullerene and pentacene as a pure organic connecting layer in tandem organic light emitting devices. J. Electrochem. Soc. 2010, 157, H69–H71. [Google Scholar] [CrossRef]
- Rand, B.P.; Burk, D.P.; Forrest, S.R. Offset energies at organic semiconductor heterojunctions and their influence on the open-circuit voltage of thin-film solar cells. Phys. Rev. B 2007, 75, 115327–1. [Google Scholar]
- Linares, M.; Beljonne, D.; Cornil, J.; Lancaster, K.; Brédas, J.; Verlaak, S.; Mityashin, A.; Heremans, P.; Fuchs, A.; et al. On the interface dipole at the pentacene-fullerene heterojunction: A theoretical study. J. Phys. Chem. C 2010, 114, 3215–3224. [Google Scholar]
- Karak, S.; Ray, S.K.; Dhar, A. Improved photovoltaic properties of pentacene/N,N'-Dioctyl-3,4,9,10-perylenedicarboximide-based organic heterojunctions with thermal annealing. Sol. Ener. Mater. Sol. Cells 2010, 94, 836–841. [Google Scholar] [CrossRef]
- Clar, E.; John, Fr. Polynuclear aromatic hydrocarbons and their derivatives. V. Naphthoanthracenes, their oxidation products and a new class of deeply colored hydrocarbons. Ber. Dtsch. Chem. Ges. 1929, 62, 3021–3029. [Google Scholar] [CrossRef]
- Clar, E.; John, Fr. Polynuclear aromatic hydrocarbons and their derivatives. VII. A new class of deeply colored radical hydrocarbons and the supposed pentacene of E. Philippi; also a reply to remarks of Roland Scholl and Oskar B.ovrddot.ottger. Ber. Dtsch. Chem. Ges. 1930, 63, 2967–2977. [Google Scholar] [CrossRef]
- Luo, J.; Hart, H. Linear acene derivatives. New routes to pentacene and naphthacene and the first synthesis of a triptycene with two anthracene moieties. J. Org. Chem. 1987, 52, 4833–4836. [Google Scholar] [CrossRef]
- Ried, W.; Anthofer, F. Einfache synthese für pentacen-6,13-chinon. Angew. Chem. 1953, 65, 601. [Google Scholar]
- Bruckner, V.; Tomasz, J. Weitere vereinfachung der pentacensynthese. Acta Chim. Acad. Sci. Hung. 1961, 28, 405–408. [Google Scholar]
- Bruckner, V.; Karczag, A.; kormendy, K.; Meszaros, M.; Tomasz, J. Einfache synthese des pentacenes. Tetrahedron Lett. 1960, 1, 5–6. [Google Scholar]
- Goodings, E.P.; Mitchard, D.A.; Owen, G. Synthesis, structure, and electrical properties of naphthacene, pentacene, and hexacene sulphides. J. Chem. Soc. PerkinTrans.I 1972, 1310–1314. [Google Scholar]
- Vets, N.; Smet, M.; Dehaen, W. Synthesis and thermolysis of a Diels-Alder adduct of pentacene and thiophosgene. Tetrahedron Lett. 2004, 45, 7287–7289. [Google Scholar]
- Sparfel, D.; Gobert, F.; Rigaudy, J. Transformations thermiques des photooxydes méso des acénes—VI: Cas des photooxydes de pentacenes. Tetrahedron 1980, 36, 2225–2235. [Google Scholar] [CrossRef]
- Chi, X. Synthesis of acenes and hydroxy-acenes. U.S. Patent 7,863,488 B2, 2011. [Google Scholar]
- Kaur, I.; Jia, W.; Kopreski, R.P.; Selvarasah, S.; Dokmeci, M.R.; Pramanik, C.; McGruer, N.E.; Miller, G.P. Substituent effects in pentacenes: Gaining control over HOMO-LUMO gaps and photooxidative resistances. J. Am. Chem. Soc. 2008, 130, 16274–16286. [Google Scholar]
- Sample Availability: Samples of pentacene prepared as described are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pramanik, C.; Miller, G.P. An Improved Synthesis of Pentacene: Rapid Access to a Benchmark Organic Semiconductor. Molecules 2012, 17, 4625-4633. https://doi.org/10.3390/molecules17044625
Pramanik C, Miller GP. An Improved Synthesis of Pentacene: Rapid Access to a Benchmark Organic Semiconductor. Molecules. 2012; 17(4):4625-4633. https://doi.org/10.3390/molecules17044625
Chicago/Turabian StylePramanik, Chandrani, and Glen P. Miller. 2012. "An Improved Synthesis of Pentacene: Rapid Access to a Benchmark Organic Semiconductor" Molecules 17, no. 4: 4625-4633. https://doi.org/10.3390/molecules17044625
APA StylePramanik, C., & Miller, G. P. (2012). An Improved Synthesis of Pentacene: Rapid Access to a Benchmark Organic Semiconductor. Molecules, 17(4), 4625-4633. https://doi.org/10.3390/molecules17044625