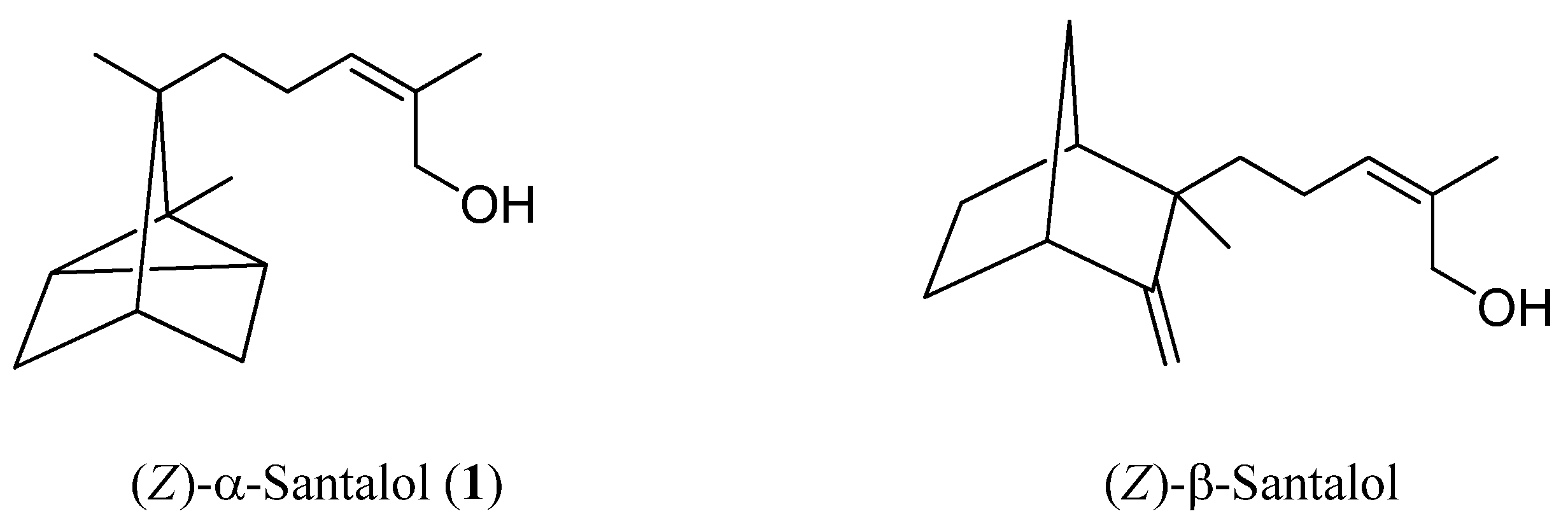
3. Experimental
3.1. General
All commercially obtained chemicals were used as received. (+)-3-Bromocamphor was purchased from Wako Pure Chemical Industries, Ltd., Japan. The solvents used in synthesis were distilled by an appropriate purification procedure. The structures of the reported compounds were determined by 1D and 2D NMR (H,H-COSY; C,H-COSY; HMBC) studies through comparison with the reported
1H-NMR and
13C-NMR spectroscopic data on α-santalol (
1) [
6]. Chemical shifts are expressed in ppm using TMS as an internal standard. NMR spectra were recorded on a AVANCE500 spectrometer (Bruker, Germany). Low-resolution mass spectrometry (MS) was performed on a JMS-700 AM spectrometer (JEOL, Japan) using electron impact (EI) ionization (70 eV), and high-resolution mass spectrometry (HRMS) was performed on a JMS-T100GCV spectrometer (JEOL, Japan) using field ionization mode. Silica Gel 60 GF254 was used for TLC. Silica Gel 60 PF254 was used for PTLC. Preparative high performance liquid chromatography (HPLC) was performed on an LC-9101 system (Japan Analytical Industry, Tokyo, Japan) equipped with a UV detector (210 nm) and 5SIL 10E column (neutral silica gel, Shodex, Tokyo, Japan; 250 mm × 10 mm i.d.; particle size: 5 μm). Gas chromatograph olfactometry (GC-O) analysis was performed on a GC-353 gas chromatograph (GL Sciences, Japan) equipped with an InertCap Pure-WAX capillary column (30 m × 0.25 mm i.d.; film thickness: 0.25 μm) and an OP 275 unit. The carrier gas was helium at a flow rate of 1 mL/min. The injections were performed in splitless mode at 250 °C. One microliter of oil solution in hexane (HPLC grade) was injected. The following temperature program was used: 40 °C for 5 min, followed by an increase to 250 °C at 6 °C/min. RIs were calculated using a series of
n-alkanes (C
16, C
17, C
18, and C
20).
3.2. Sensory Evaluation
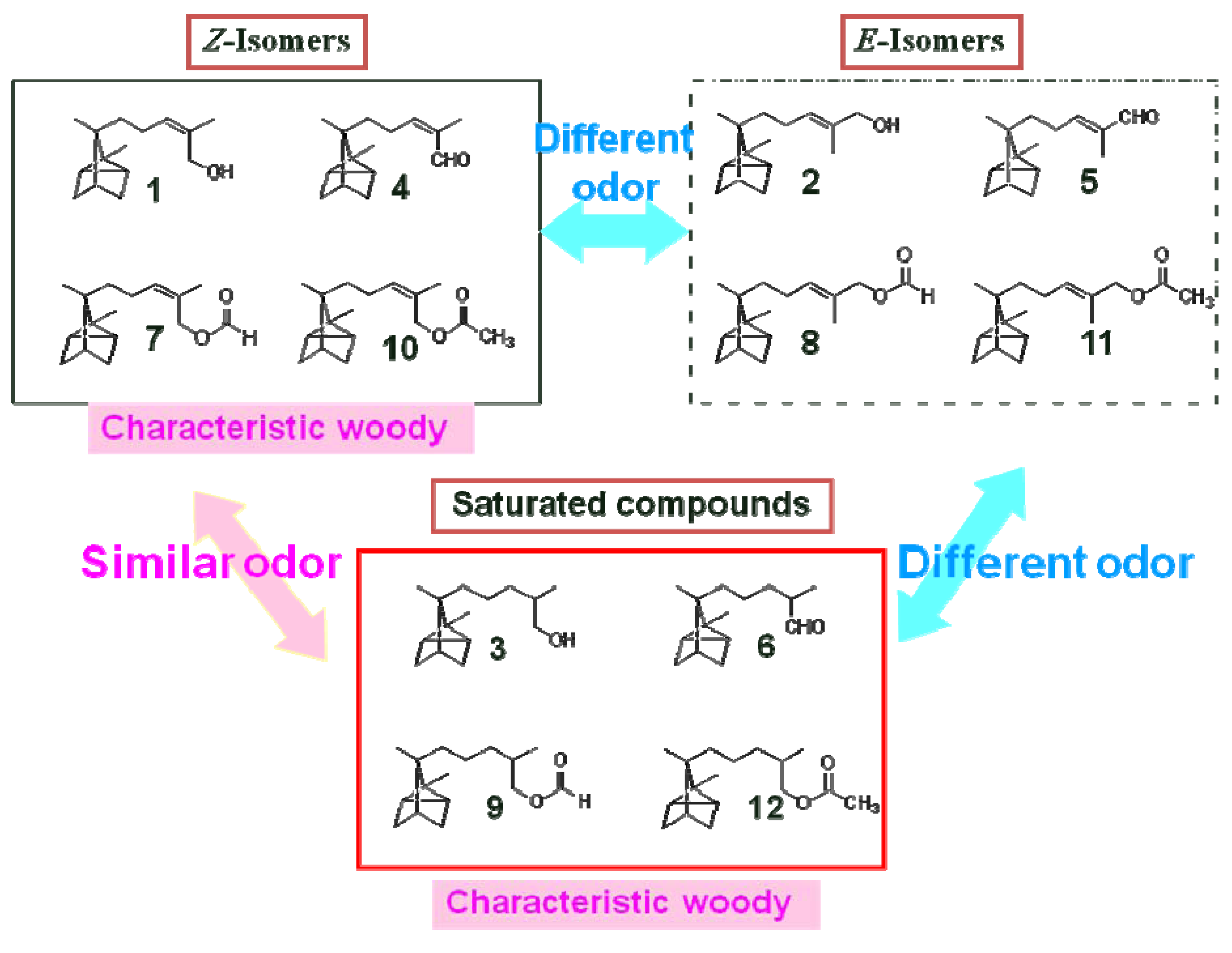
Sensory evaluation of the synthesized compounds without solvent was performed by an expert panel consisting of four members of Yamada-matsu Co., Ltd. The similarities and differences in odor character of the compounds were evaluated. The sensory evaluation was also performed by a non-expert panel consisting of untrained participants. The sensory evaluation results were consistent between the expert and non-expert panels.
3.2. Synthesis of a-Santalyl Benzyl Ethers and Separation of Their (Z)- and (E)-Isomers
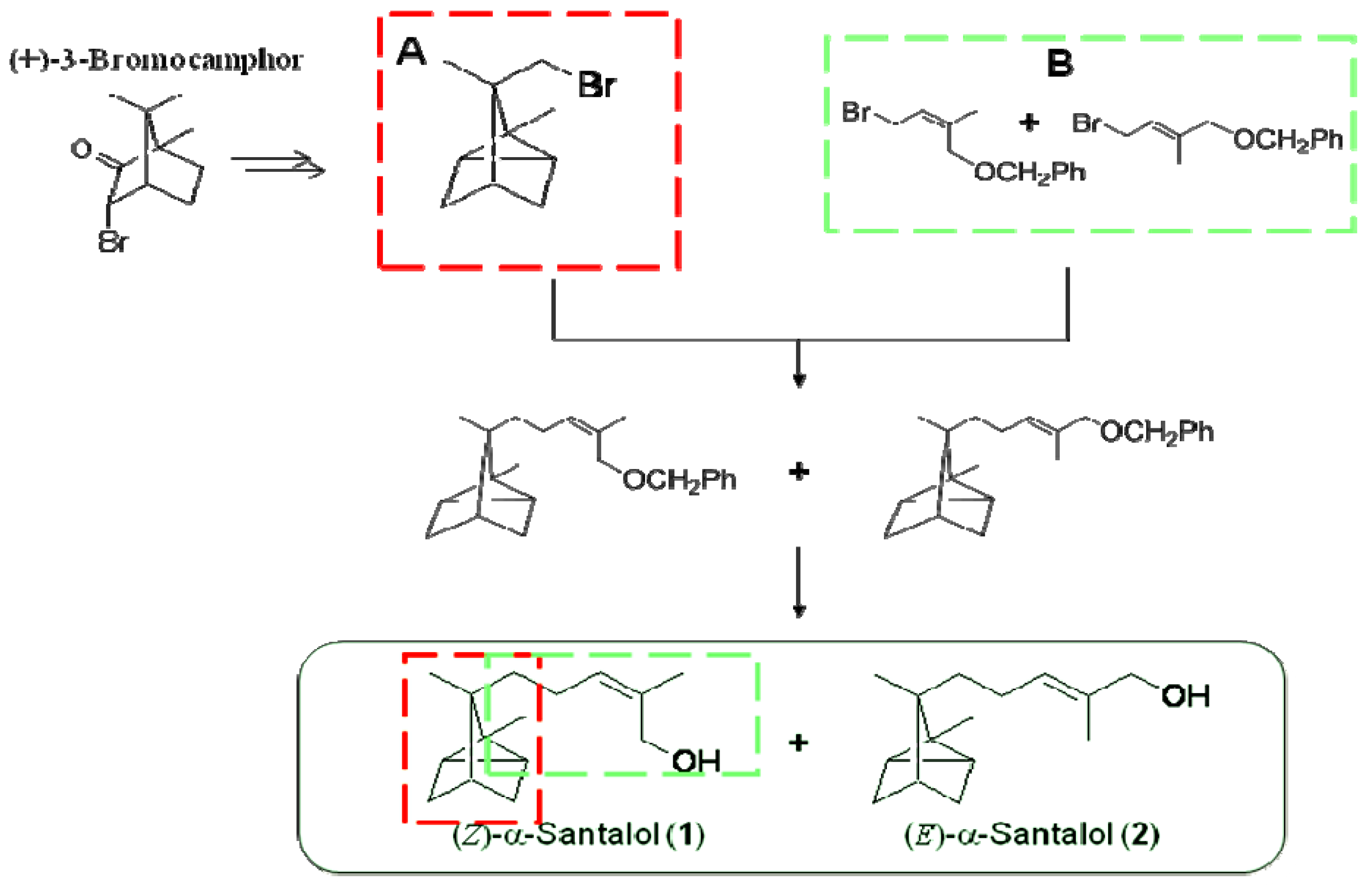
A solution of t-BuLi in pentane (1.60 mol/L, 20.0 mL, 32.0 mmol) was cooled to −78 °C in a dry ice-methanol bath under a nitrogen atmosphere. An absolute ether solution (40 mL) of (−)-8-bromotricyclene (3.03 g, 14.1 mmol) was added dropwise to the solution over 1.5 h. After 10 min, the reaction mixture was warmed to 25 °C and stirred for 2 h. The reaction mixture was again cooled to −78 °C, and ether solution of 1-benzyloxy-4-bromo-2-methyl-2-butene (2.02 g, 7.92 mmol; E/Z-isomer = 62:38) was added dropwise to the solution over 1 h. The reaction mixture was warmed to 25 °C and held at that temperature. After 12 h the completion of the reaction was observed by TLC [(SiO2, hexane-AcOEt (95:5)]. Saturated ammonium chloride solution (20 mL) and ether (150 mL) were added to the reaction mixture. The ether solution was washed with saturated ammonium chloride solution (20 mL × 2) and saturated sodium chloride solution (20 mL × 2). The solution was then dried over anhydrous magnesium sulfate. Removal of the solvent under reduced pressure gave an oil. The oil was purified by bulb-to-bulb distillation (130–140 °C at 0.06 Torr) to afford α-santalyl benzyl ethers (1.03 g, 42%; E/Z-isomer = 62:38) as a colorless oil. The E/Z-mixture was separated by HPLC and isolated as colorless oils.
(Z)-Benzyl ethers. Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.82−0.90 (m, 3H, H-2′,6′), 0.86 (s, 3H, H-9′), 0.98 (s, 3H, H-8′), 1.03−1.08 (m, 1H, H-4′), 1.11−1.16 (m, 2H, H-3′,5′), 1.25−1.30 (m, 5H, H-5), 1.51−1.60 (m, 2H, H-3′,5′), 1.79 (s, 3H, H-6), 1.95−2.00 (m, 2H, H-4), 4.01 (s, 2H, H-1), 4.45 (s, 2H, H-1′′), 5.39 (t, 1H, H-3, J = 7.0 Hz), 7.33−7.36 (m, 5H, H-2′′−5′′); 13C-NMR (125 MHz, CDCl3) δ 10.65 (C-8′), 17.51 (C-9′), 19.49 (C-2′), 19.52 (C-6′), 21.25 (C-6), 22.93 (C-4), 27.37 (C-1′), 31.12 (C-5′), 31.51 (C-3′), 35.00 (C-5), 38.17 (C-4′), 45.87 (C-7′), 61.60 (C-1), 129.53 (C-3), 133.67 (C-2).
(E)-Isomer of the benzyl ethers. Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.75−0.90 (m, 2H, H-2′,6′), 0.87 (s, 3H, H-9′), 1.00 (s, 3H, H-8′), 1.03−1.08 (m, 1H, H-4′), 1.11−1.20 m, (2H, H-3′,5′), 1.24−1.27 (m, 2H, H-5), 1.51−1.60 (m, 2H, H-3′,5′), 1.68 (s, 3H, H-6), 1.94−2.03 (m, 2H, H-4), 3.90 (s, 2H, H-1), 4.45 (s, 2H, H-1′′), 5.43 (t, 1H, H-3, J = 7.0 Hz), 7.34−7.36 (m, 5H, H-2′′−5′′); 13C-NMR (125 MHz, CDCl3) δ 10.65 (C-8′), 13.53 (C-6), 17.51 (C-9′), 19.51 (C-2′), 19.56 (C-6′), 22.91 (C-4), 27.41 (C-1′), 31.01 (C-5′), 31.52 (C-3′), 34.22 (C-5), 38.19 (C-4′), 45.87 (C-7′), 69.13 (C-1), 1297.34 (C-3), 134.14 (C-2).
3.3. Synthesis of α-Santalol from α-Santalyl Benzyl Ether and Separation of Its (Z)-Isomer (1) and (E)-Isomer (2)
A round-bottom flask was cooled to −78 °C in a dry ice-methanol bath under nitrogen atmosphere. Ethylamine (dried with potassium hydroxide; 19 mL) was added to the flask. Flakes of lithium were added to the ethylamine, and after 10 min, the solution became deep blue. After the solution was stirred for 40 min, a hexane solution (8 mL) of α-santalyl benzyl ether (832 mg, 2.68 mmol) (E/Z-isomer = 62:38) was added dropwise over 30 min. The progress of the reaction was monitored by TLC [(SiO2, hexane-AcOEt (10:3)]. Ammonium chloride solution was added to the reaction mixture until the blue color disappeared. Methanol (10 mL) was added, and ethylamine was removed under reduced pressure from the reaction mixture at room temperature. The solution was extracted with ether (50 mL × 3). The obtained solution was washed with saturated sodium chloride solution (20 mL × 3). The organic solution was dried over anhydrous magnesium chloride, and removal of the solvent gave a crude mixture, which was purified by column chromatography [SiO2, hexane-AcOEt (10:3)] to give the E/Z-mixture of α-santalol (331 mg, 56%; E/Z-isomer = 62:38) as a colorless liquid. Repeated purification by PTCL [SiO2; first, hexane-AcOEt (70:30); second, hexane-isopropanol (90:10)] afforded (Z)-α-santalol (1; 88.3 mg) and (E)-α-santalol (1; 75.1 mg) as pure colorless oils.
(Z)-α-Santalol (1). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.82−0.85 (m, 2H, H-2′,6′), 0.83 (s, 3H, H-9′), 0.99 (s, 3H, H-8′), 1.03−1.08 (m, 2H, H-3′,5′), 1.11−1.17 (m, 1H, H-5), 1.20−1.27 (m, 1H, H-5), 1.55−1.62 (m, 3H, H-3′,4′,5′), 1.79 (s, 3H, H-6), 1.94−2.00 (m, 2H, H-4), 4.14 (s, 2H, H-1), 5.31 (t, 1H, J = 7.5 Hz, H-3); 13C-NMR (125 MHz, CDCl3) δ 10.65 (C-8′), 17.51 (C-9′), 19.49 (C-2′), 19.52 (C-6′), 21.25 (C-6), 22.93 (C-4), 27.37 (C-1′), 31.12 (C-5′), 31.51 (C-3′), 35.00 (C-5), 38.17 (C-4′), 45.87 (C-7′), 61.60 (C-1), 129.53 (C-3), 133.67 (C-2).
(E)-α-Santalol (2). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.84−0.90 (m, 2H, H-2′,6′), 0.87 (s, 3H, H-9′), 1.00 (s, 3H, H-8′), 1.02−1.08 (m, 2H, H-3′,5′), 1.12−1.20 (m, 1H, H-5), 1.22−1.35 (m, 1H, H-5), 1.57−1.63 (m, 3H, H-3′,4′,5′), 1.67 (s, 3H, H-6), 1.92−2.02 (m, 2H, H-4), 4.00 (s, 2H, H-1), 5.41 (t, 1H, J = 7.0 Hz, H-3); 13C-NMR (125 MHz, CDCl3) δ 10.65 (C-8′), 13.53 (C-6), 17.51 (C-9′), 19.51 (C-2′), 19.56 (C-6′), 22.91 (C-4), 27.41 (C-1′), 31.01 (C-5′), 31.52 (C-3′), 34.22 (C-5), 38.19 (C-4′), 45.87 (C-7′), 69.13 (C-1), 127.34 (C-3), 134.14 (C-2).
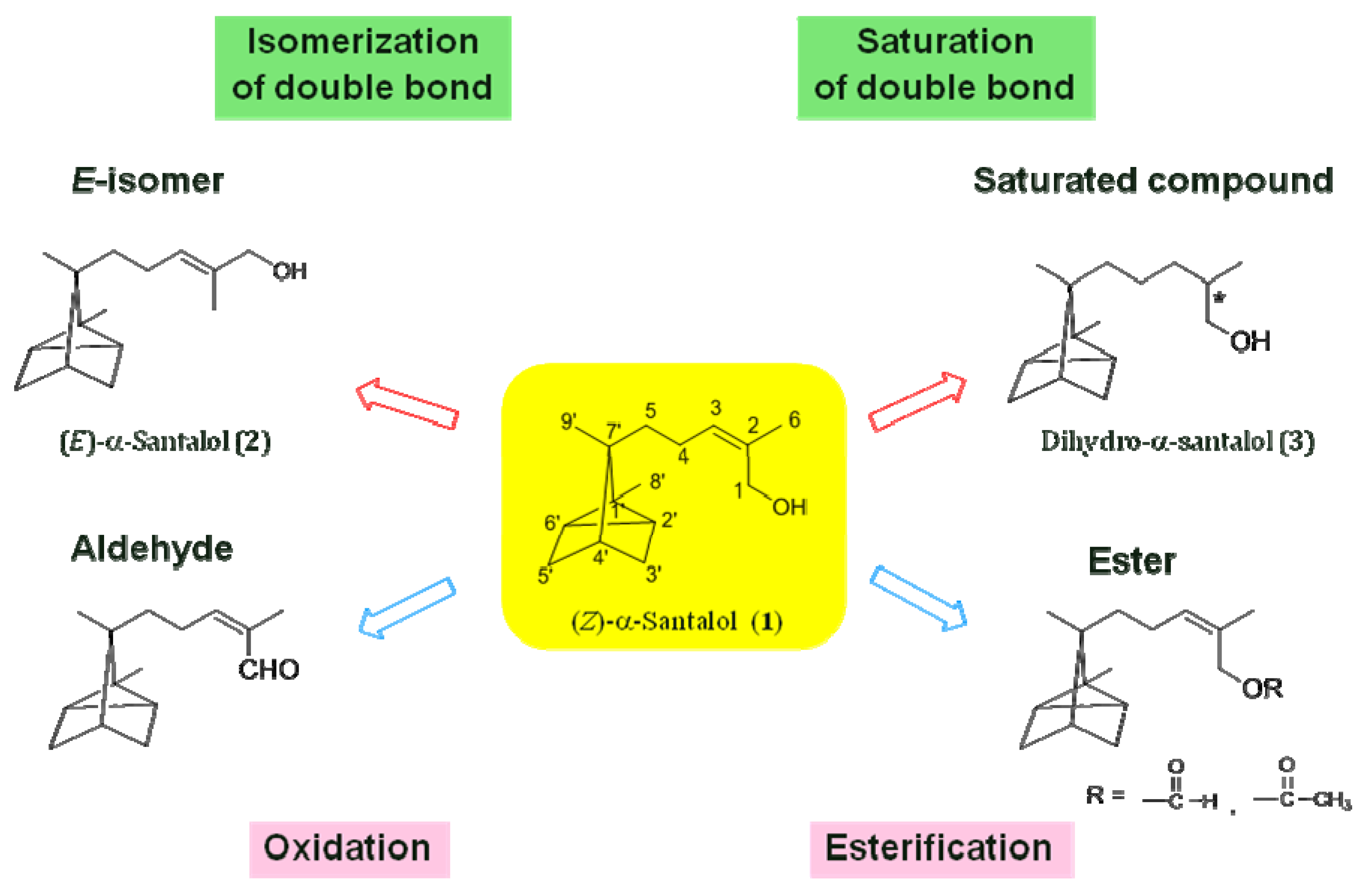
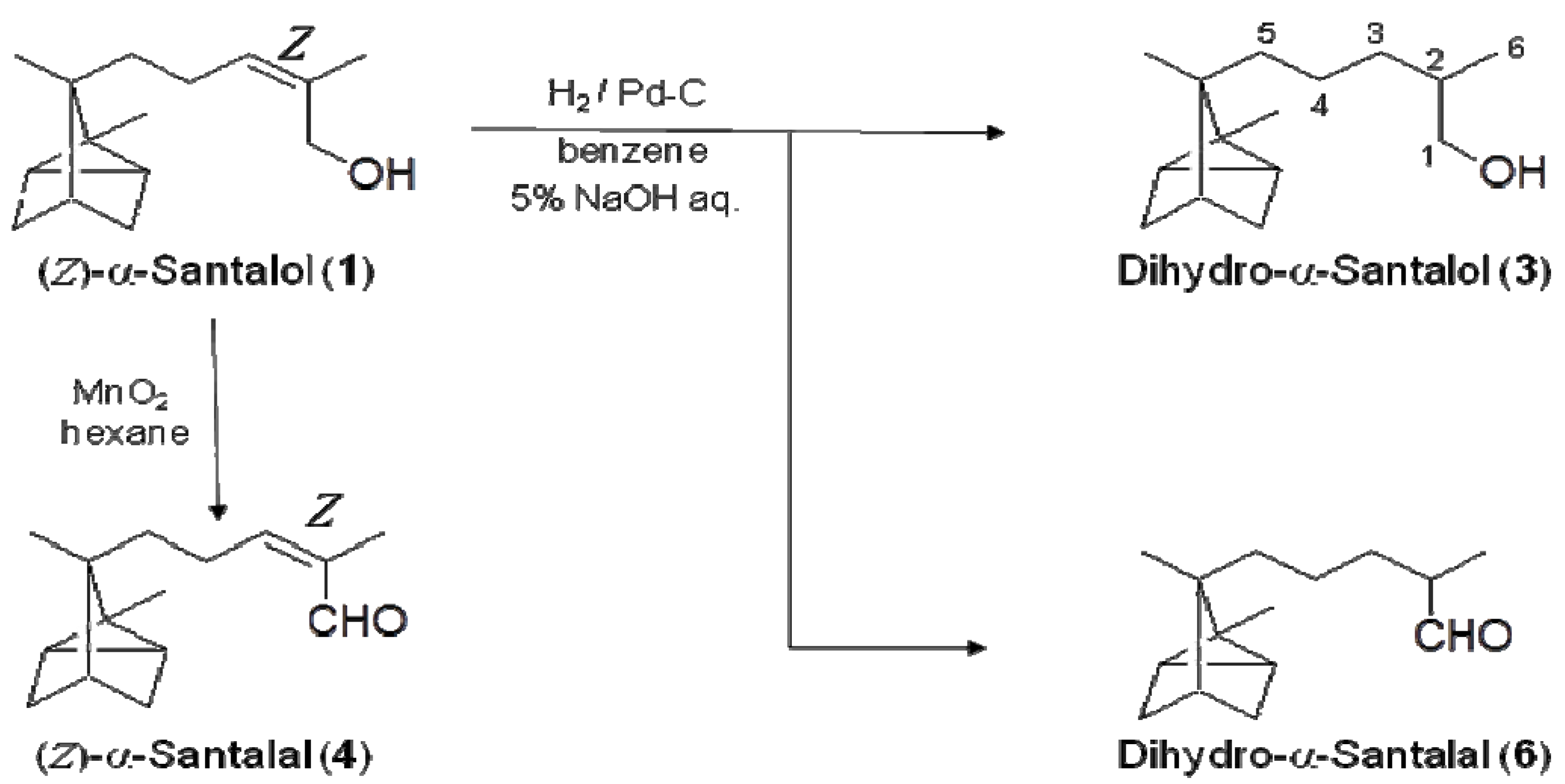
3.4. Synthesis of Dihydro-α-Santalol (3) and Dihydro-α-Santalal (6)
To a flask that had been purged with nitrogen, 5% Pd-C (22.5 mg) was added. The E/Z-mixture of α-santalol (E/Z-isomer = 62:38; in 1 mL of benzene), benzene (2 mL), and 5% aqueous sodium hydroxide solution (1 mL) were added to the flask. Hydrogen was introduced to the flask, and the solution was stirred under hydrogen atmosphere for 8 h at room temperature. The progress of the reaction was monitored by TLC (SiO2, CHCl3). Pd-C was filtered from the solution, and removal of the solvent gave a crude oil. The mixture was purified by PTLC (SiO2, CHCl3) to afford dihydro-α-santalol (3; 5.7 mg, 25%) and dihydro-α-santalal (6; 4.7 mg, 21%).
Dihydro-α-santalol (3). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.79−0.88 (m, 2H, H-2′,6′), 0.82 (s, 3H, H-9′), 0.92 (d, 3H, J = 6.6 Hz, H-6), 0.99 (s, 3H, H-8′), 1.01−1.06 (m, 2H, H-3′,5′), 1.10–1.38 (m, 6H, H-3,4,5), 1.54−1.67 (m, 3H, H-3′,4′,5′), 1.68−1.81 (m, 1H, H-2), 3.42 (dd, 1H, J = 10.5, 6.5 Hz, H-1), 3.51 (dd, 1H, J = 10.5, 6.5 Hz, H-1); 13C-NMR (125 MHz, CDCl3) δ 10.65 (C-8′), 16.57 and 16.64 (C-6), 17.61 (C-9′), 19.52 (C-2′,6′), 21.81 and 21.85 (C-4), 27.37 (C-1′), 31.02 (C-5′), 31.48 (C-3′), 34.19 and 34.20 (C-3), 34.54 (C-5), 35.68 and 35.70 (C-2), 38.23 (C-4′), 45.84 (C-7′), 68.44 and 68.52 (C-1).
Dihydro-α-santalal (6). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.79−0.88 (m, 2H, H-2′,6′), 0.86 (s, 3H, H-9′), 1.09 (d, 3H, J = 7.0 Hz, H-6), 1.12−1.43 (m, 10H), 1.50−1.62 (m, 3H), 1.65–1.75 (m, 1H), 2.30–2.38 (m, 1H, H-2), 9.62 (d, 1H, J = 3.5 Hz, H-1); 13C-NMR (125 MHz, CDCl3) δ 10.7 (C-8′), 13.35 and 13.41 (C-6), 17.6 (C-9′), 19.5 (C-2′, 6′), 21.94 and 21.96 (C-4), 27.4 (C-1′), 31.0 (C-5′ or C-3′), 31.5 (C-3′ or C-5′), 31.58 and 31.63 (C-3), 34.4 (C-5), 38.2 (C-4′), 45.8 (C-7′), 46.31 and 46.37 (C-2), 205.4 (C-1) MS (m/z, %) 220 (M+, 7), 187 (31), 171 (21), 153 (49), 131 (97), 121 (91), 93 (100), 87 (98), 79 (25), 77 (16). HRMS (m/z): [M]+ calcd for C15H24O, 220.1827; found, 220.1822.
3.5. Synthesis of Aldehyde Derivatives of α-Santalol
(Z)-α-Santalol (1; 8.0 mg, 0.036 mmol) was dissolved in hexane (0.5 mL). Manganese dioxide (70.8 mg) was added to the solution and stirred for 24 h. The progress of the reaction was monitored by TLC (SiO2, CHCl3). Hexane (5 mL) was added to the reaction mixture, and the solid was filtered and washed with chloroform (5 mL). Removal of organic solvent from the obtained solution gave (Z)-α-santalal (4; 7.1 mg, 96%) as a colorless oil. Through a similar procedure, (E)-α-santalal (5; 8.0 mg, 80%) was synthesized from (E)-α-santalol (2; 10.0 mg, 0.045 mmol).
(Z)-α-Santalal (4). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.80−0.92 (m, 2H, H-2′,6′), 0.86 (s, 3H, H-9′), 1.01 (s, 3H, H-8′), 0.99−1.12 (m, 3H, H-3′,4′,5′), 1.24−1.42 (m, 2H, H-5), 1.57−1.65 (m, 2H, H-3′,5′), 1.77 (s, 3H, H-6), 2.44−2.55 (m, 2H, H-4), 6.54 (t, 1H, H-3, J = 8.4 Hz), 10.16 (s, 1H, H-1); 13C-NMR (125 MHz, CDCl3) δ 10.65 (C-8′), 17.51 (C-9′), 19.49 (C-2′), 19.52 (C-6′), 21.25 (C-6), 22.93 (C-4), 27.37 (C-1′), 31.12 (C-5′), 31.51 (C-3′), 35.00 (C-5), 38.17 (C-4′), 45.87 (C-7′), 61.60 (C-1), 129.53 (C-3), 133.67 (C-2).
(E)-α-Santalal (5). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.80−0.92 (m, 2H, H-2′,6′), 0.86 (s, 3H, H-9′), 1.00 (s, 3H, H-8′), 1.04−1.16 (m, 3H, H-3′,4′,5′), 1.25−1.44 m, (2H, H-5), 1.57−1.63 (m, 2H, H-3′,5′), 1.75 (s, 3H, H-6), 2.23−2.35 (m, 2H, H-4), 6.50 (t, 1H, H-3, J = 7.5 Hz), 9.39 (s, 1H, H-1); 13C-NMR (125 MHz, CDCl3) δ 10.65 (C-8′), 13.53 (C-6), 17.51 (C-9′), 19.51 (C-2′), 19.56 (C-6′), 22.91 (C-4), 27.41 (C-1′), 31.01 (C-5′), 31.52 (C-3′), 34.22 (C-5), 38.19 (C-4′), 45.87 (C-7′), 69.13 (C-1), 1297.34 (C-3), 134.14 (C-2).
3.6. Synthesis of Formates of α-Santalol
(Z)-α-Santalol (1; 6.2 mg, 0.028 mmol) was dissolved in absolute benzene (1.0 mL). Formic acid (30 μL, 0.78 mmol) and anhydrous magnesium sulfate (87 mg) were added to the solution and stirred overnight. The reaction was monitored by TLC (SiO2, CHCl3). A large amount of 1 was not consumed. Formic acid (30 μL, 0.78 mmol) and anhydrous magnesium sulfate (142 mg) were also added to the solution. The solution was stirred overnight, and the progress of the reaction was monitored by TLC. The reaction mixture was extracted with benzene (5 mL × 4). The solution was washed with saturated sodium hydrogen carbonate solution (2 mL × 2) and saturated sodium chloride solution (2 mL × 2). The obtained organic solution was dried over anhydrous magnesium sulfate. Removal of the solvent from the reaction mixture gave (Z)-α-santalyl formate (7; 6.9 mg, 99%) as a colorless oil. In a similar procedure, the reaction of (E)-α-santalol (2; 9.2 mg, 0.042 mmol) and dihydro-α-santalol (3; 6.5 mg, 0.029 mmol) gave (E)-α-santalyl formate (8; 10.2 mg, 98%) and dihydro-α-santalyl formate (9; 7.1 mg, 97%), respectively.
(Z)-α-Santalyl formate (7). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.82−0.88 (m, 2H, H-2′,6′), 0.83 (s, 3H, H-9′), 0.99 (s 3H, H-8′), 1.04−1.08 (m, 2H, H-3′,5′), 1.12−1.18 (m, 1H, H-5), 1.23−1.33 (m, 1H, H-5), 1.55−1.62 (m, 3H, H-3′,4′,5′), 1.78 (s, 3H, H-6), 1.96−2.05 (m, 2H, H-4), 4.69 (s, 2H, H-1), 5.43 (t, 1H, J = 7.5 Hz, H-3), 8.11 (s, 1H, -CHO); 13C-NMR (125 MHz, CDCl3) δ 10.64 (C-8′), 17.49 (C-9′), 19.49 (C-2′), 19.53 (C-6′), 21.41 (C-6), 23.18 (C-4), 29.39 (C-1′), 30.99 (C-5′), 31.51 (C-3′), 34.62 (C-5), 38.16 (C-4′), 45.87 (C-7′), 62.55 (C-1), 128.47 (C-3), 132.53 (C-2), 161.12 (-CHO).
(E)-α-Santalyl formate (8). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.83−0.89 (m, 2H, H-2′,6′), 0.84 (s, 3H, H-9′), 1.00 (s, 3H, H-8′), 1.02−1.08 (m, 2H, H-3′,5′), 1.12−1.19 (m, 1H, H-5), 1.23−1.29 (m, 1H, H-5), 1.56−1.63 (m, 3H, H-3′,4′,5′), 1.76 (s, 3H, H-6), 1.94−2.02 (m, 2H, H-4), 4.55 (s, 2H, H-1), 5.51 (t, 1H, H-3, J = 7.3 Hz), 8.10 (s, 1H, -CHO); 13C-NMR (125 MHz, CDCl3) δ 10.64 (C-8′), 13.75 (C-6), 17.49 (C-9′), 19.50 (C-6′), 19.56 (C-2′), 223.12 (C-4), 27.40 (C-1′), 31.01 (C-3′), 31.52 (C-5′), 33.91 (C-5), 38.19 (C-4′), 45.86 (C-7′), 69.91 (C-1), 128.79 (C-3), 131.66 (C-2), 161.02 (-CHO); MS (m/z, %) 248 (M+, 1), 202 (36), 187 (20), 135 (10), 121 (35), 107 (40), 93 (100), 91 (73), 79 (50), 77 (49). HRMS (m/z): [M]+ calcd for C16H24O2, 240.1776; found, 248.1779.
Dihydro-α-santalyl formate (9). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.80−0.89 (m, 2H, H-2′,6′), 0.83 (s, 3H, H-8′), 0.95 (d, 3H, J = 6.5 Hz, H-6), 0.99 (s, 3H, H-8′), 1.01−1.06 m, (2H, H-3′,5′), 1.11−1.37 (m, 6H, H-3,4,5), 1.53−1.67 (m, 3H, H-3′,4′,5′), 1.80−1.85 (m, 1H, H-2), 3.96 (dd, 1H, J = 10.8, 6.8 Hz, H-1), 4.06 (dd, 1H, J = 10.8, 5.8 Hz, H-1), 8.09 (s, 1H, -CHO); 13C-NMR (125 MHz, CDCl3) δ 10.68 (C-8′), 16.78 and 16.86 (C-6), 17.60 (C-9′), 19.52 (C-2′,6′), 21.67 and 21.69 (C-4), 27.38 (C-1′), 32.40 (C-3), 31.02 (C-5′), 31.48 (C-3′), 34.30 (C-5), 34.32 and 34.45 (C-2), 38.22 (C-4′), 45.83 (C-7′), 68.91 and 68.96 (C-1), 161.28 (-CHO); MS (m/z, %) 250 (M+, 42), 205 (6), 121 (100), 93 (86), 91 (25), 79 (22), 77 (14). HRMS (m/z): [M]+ calcd for C16H26O2, 250.1933; found, 250.1934.
3.7. Synthesis of Acetates of α-Santalol
(Z)-α-Santalol (1; 5.7 mg, 0.026 mmol), triethylamine (0.4 mL), anhydrous acetic acid (0.020 mL, 0.21 mmol), and dimethylaminopyridine (2.4 mg) were added to a flask and stirred for 12 h. The progress of the reaction was monitored by TLC (SiO2, chloroform). The reaction mixture was diluted with hexane (30 mL). The solvent was washed with 1 mol/L HCl (3 mL × 2), saturated sodium hydrogen carbonate solution (3 mL × 2), and saturated sodium chloride solution (3 mL × 2). The solution was dried over anhydrous magnesium sulfate. Removal of the organic solvent gave (Z)-santalyl acetate (10; 5.8 mg, 85%) as a colorless oil. The (E)-isomer (11) and dihydro derivative (12) were synthesized from (E)-α-santalol (2) and dihydro-α-santalol (2), respectively, through the same procedure; The acetate (6.4 mg, 79%) was synthesized from (E)-α-santalol (6.6 mg, 0.030 mol), and the acetate (2.3 mg, 96%) was synthesized and from dihydro-α-santalol (2.0 mg, 0.0091 mmol).
(Z)-α-Santalyl acetate (10). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.82 (s, 3H, H-9′), 0.83−0.87 (m, 2H, H-2′,6′), 0.99 (s, 3H, H-8′), 1.03−1.07 (m, 2H, H-3′,5′), 1.12−1.18 (m, 1H, H-5), 1.21−1.28 (m, 1H, H-5), 1.55−1.62 (m, 3H, H-3′,4′,5′), 1.74 (s, 3H, H-6), 1.96−2.06 (m, 2H, H-4), 2.07 (s, 3H, COCH3), 4.59 (s, 2H, H-1), 5.41 (t, 1H, J = 7.5 Hz, H-3); 13C-NMR (125 MHz, CDCl3) δ 10.64 (C-8′), 17.49 (C-9′), 19.49 (C-2′), 19.53 (C-6′), 20.98 (COCH3), 21.47 (C-6), 23.13 (C-4), 27.38 (C-1′), 30.99 (C-5′), 31.50 (C-3′), 34.66 (C-5), 38.15 (C-4′), 45.86 (C-7′), 63.19 (C-1), 129.15 (C-3), 131.87 (C-2), 171.21 (-CO-); MS (m/z, %) 262 (M+, 1), 202 (58), 187 (23), 135 (19), 121 (88), 107 (44), 94 (100), 93 (97), 79 (36), 77(24). HRMS (m/z): [M]+ calcd for C17H26O2, 262.1933; found, 262.1937.
(E)-α-Santalyl acetate (11). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.83−0.88 (m, 2H, H-2′,6′), 0.84 (s, 3H, H-9′), 1.00 (s, 3H, H-8′), 1.02−1.08 (m, 2H, H-3′,5′), 1.13−1.19 (m, 1H, H-5), 1.23−1.29 (m, 1H, H-5), 1.57−1.62 (m, 3H, H-3′,4′,5′), 1.67 (s, 3H, H-6), 1.94−2.00 (m, 2H, H-4), 2.08 (s,3H, COCH3), 4.45 (s, 2H, H-1), 5.41 (t, 1H, J = 7.0 Hz, H-3); 13C-NMR (125 MHz, CDCl3) δ 10.64 (C-8′), 13.81 (C-6), 17.48 (C-9′), 19.50 (C-2′), 19.55 (C-6′), 21.04 (COCH3), 23.06 (C-4), 27.39 (C-1′), 31.00 (C-5′), 31.51 (C-3′), 33.97 (C-5), 38.17 (C-4′), 45.85 (C-7′), 70.40 (C-1), 129.39 (C-3), 130.73 (C-2), 171.06 (-CO-); MS (m/z, %) 262 (M+, 1), 202 (97), 187 (43), 135 (31), 121 (99), 107 (74), 94 (100), 93 (99), 79 (58), 77 (37). HRMS (m/z): [M]+ calcd for C17H26O2, 262.1933; found, 262.1937.
Dihydro-α-santalyl acetate (12). Colorless liquid; 1H-NMR (500 MHz, CDCl3) δ 0.79−0.88 (m, 2H, H-2′,6′), 0.86 (s, 3H, H-9′), 0.94 (d, 3H, J = 6.5 Hz, H-6), 0.98−1.45 (m, 12H), 1.52−1.67 (m, 3H), 2.06 (s, 3H, -COCH3), 3.84 (dd, 1H, J = 11.0, 6.5 Hz, H-1), 3.95 (dd, 1H, J = 11.0, 6.5 Hz, H-1); 13C-NMR (125 MHz, CDCl3) δ 10.69 (C-8′), 16.86 (C-6), 17.60 (C-9′), 19.51 (C-2′,6′), 20.99 (COCH3), 21.68 (C-4), 27.77 (C-1′), 31.01 (C-5′), 31.47 (C-3′), 32.53 and 32.42 (C-3), 33.43 (C-5), 34.46 (C-2), 38.21 (C-4′), 44.20 (C-7′), 69.53 and 69.63 (C-1), 171.34 (-OCO-); MS (m/z, %) 264 (M+, 34), 249 (2), 221 (3), 135 (11), 121 (100), 119 (18), 93 (72), 91 (18), 79 (16), 77 (14). HRMS (m/z): [M]+ calcd for C17H28O2, 260.2089; found, 260.2090.