A Novel Class of Selective Acetylcholinesterase Inhibitors: Synthesis and Evaluation of (E)-2-(Benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles
Abstract
:1. Introduction
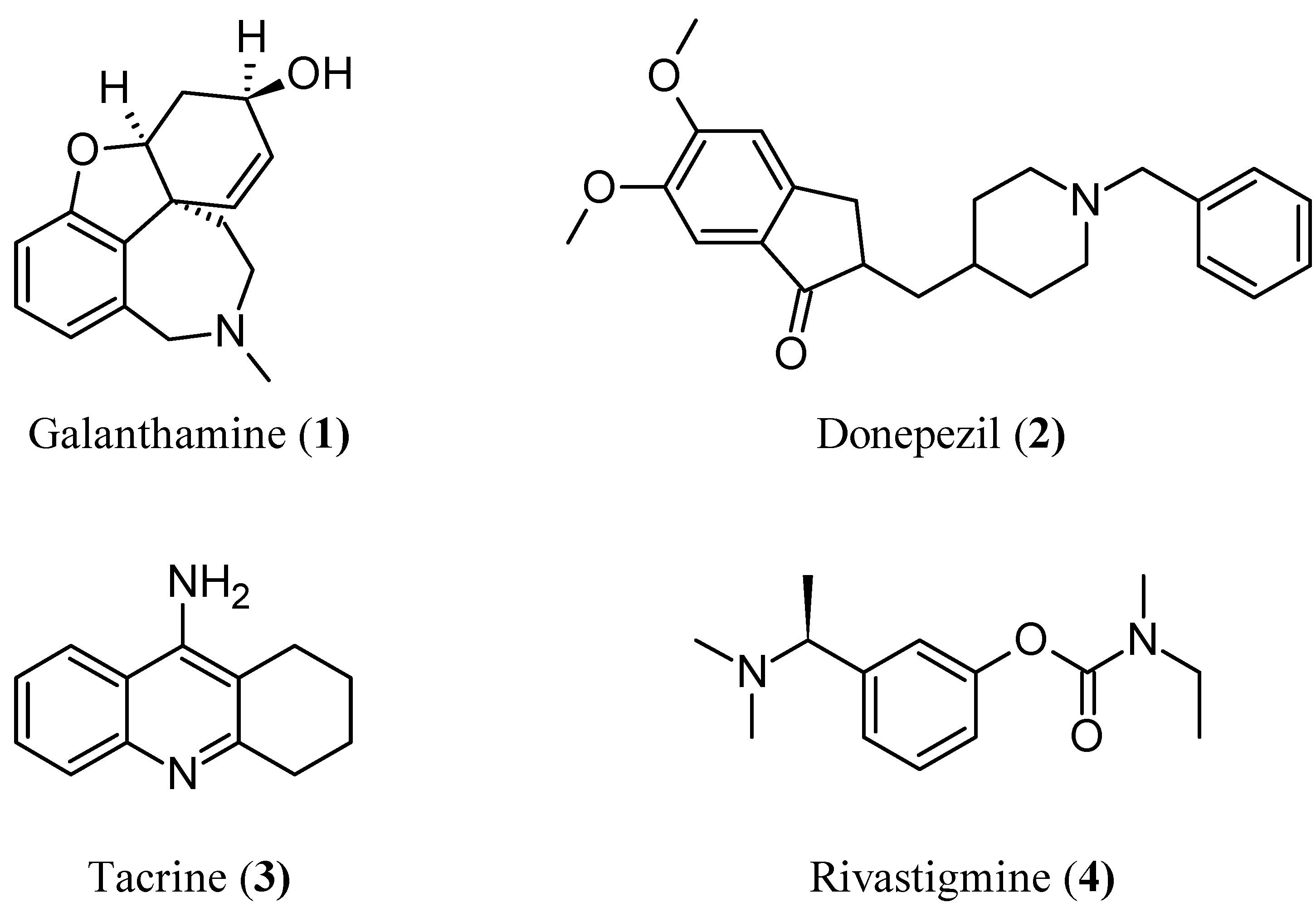
2. Results and Discussion
2.1. Chemistry
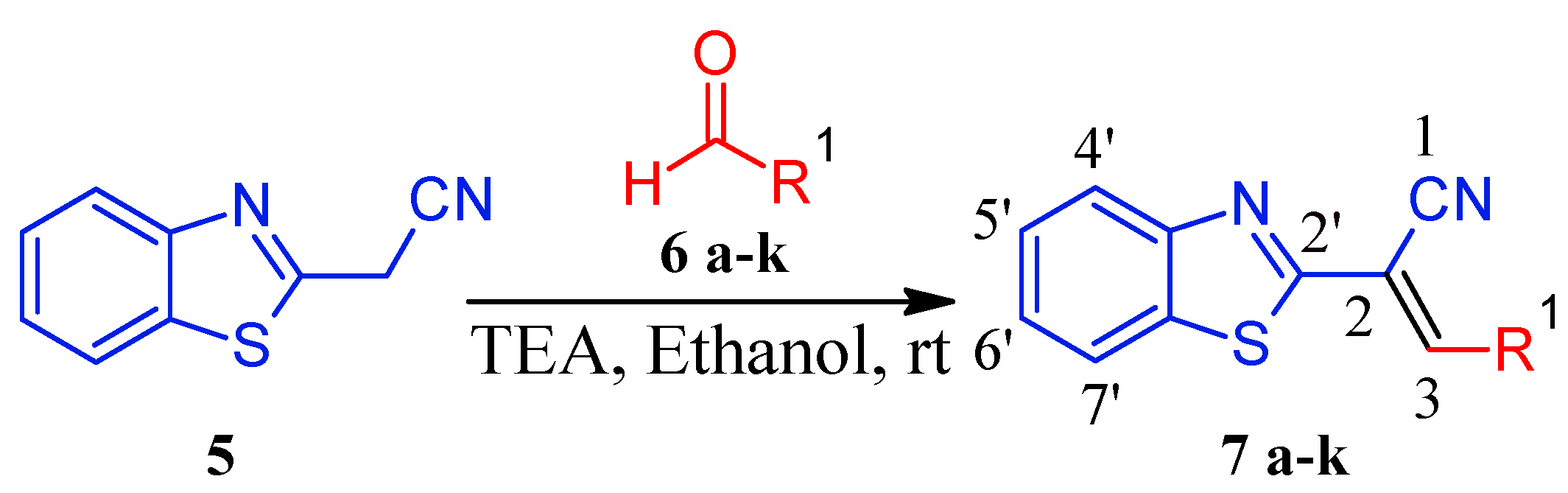
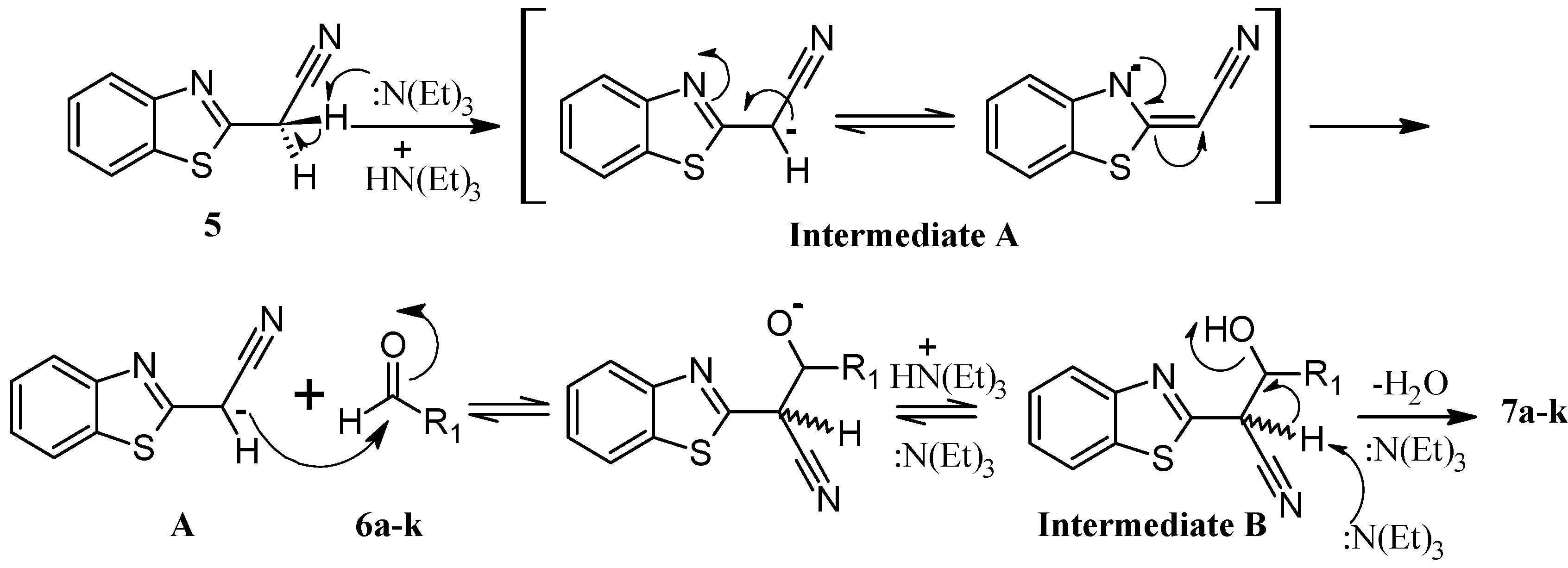
2.2. Biological Activities
| Entry | R1 | mp (°C) | Yield (%) | Time reaction (min) | IC50a (µM) | Selectivity for AChE | ||
|---|---|---|---|---|---|---|---|---|
| AChE | BuChE | |||||||
| 7a |  | 209–211 | 84 | 18 | 1274 ± 0.7 | >1870.5 ± 0.6 c | ||
| 7b |  | 241–243 | 87 | 15 | 1179 ± 0.5 | 1396.60 ± 0.8 | ||
| 7c |  | 228–230 | 85 | 17 | 699 ± 0.3 | >1438.3 ± 1.0 c | ||
| 7d |  | 244–246 | 40 | 21 | 504 ± 1.0 | >1439.14 ± 1.0 c | ||
| 7e |  | 190–192 | 80 | 40 | 303 ± 0.8 | >1981.8 ± 0.9 c | ||
| 7f |  | 155–157 | 66 | 18 | 103 ± 0.7 | >2286.3 ± 0.7 c | 22.20 | |
| 7g |  | 185–187 | 59 | 25 | 64 ± 0.6 | 474.05 ± 0.8 | 7.40 | |
| 7h |  | 188–190 | 56 | 26 | >1326.7 ±0.7 c | >1326.75 ± 1.0 c | ||
| 7i |  | 138–140 | 51 | 22 | >1981.80 ± 1.0 c | 1883 ± 0.8 | ||
| 7j |  | 191–193 | 84 | 20 | >1477.45 ± 1.0 c | 1273 ± 0.7 | ||
| 7k |  | 158–160 | 83 | 14 | >1898.90 ± 0.8 c | >1899 ± 0.8 c | ||
| Galanthamine | 0.54 ± 0.7 | 8.80 ± 0.5 | 16.29 | |||||
2.3. Kinetic Study

2.4. Molecular Docking

3. Experimental
3.1. General
3.2. Pharmacology
3.2.1. AChE Assay
3.2.2. Kinetic Characterization of AChE Inhibition
3.2.3. Molecular Docking
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 3a-k are available from the authors.
References
- Greenblatt, H.M.; Guillou, C.; Guénard, D.; Argaman, A.; Botti, S.; Badet, B.; Thal, C.; Silman, I.; Sussman, J.L. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: Implications for structure-based drug design. J. Am. Chem. Soc. 2004, 126, 15405–15411. [Google Scholar]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. J. Am. Med. Assoc. 1994, 271, 992–998. [Google Scholar]
- Bar-On, P.; Millard, C.B.; Harel, M.; Dvir, H.; Enz, A.; Sussman, J.L.; Silman, I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry 2002, 41, 3555–3564. [Google Scholar]
- Raves, M.L.; Harel, M.; Pang, Y.P.; Silman, I.; Kozikowski, A.P.; Sussman, J.L. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (−)-huperzine A. Nat. Struct. Biol. 1997, 4, 57–63. [Google Scholar] [CrossRef]
- Cheng, Y.; An, L.-K.; Wu, N.; Wang, X.-D.; Bu, X.-Z.; Huang, Z.-S.; Gu, L.-Q. Synthesis, cytotoxic activities and structure-activity relationships of topoisomerase I inhibitors: indolizinoquinoline-5,12-dione derivatives. Bioorg. Med. Chem. 2008, 16, 4617–4625. [Google Scholar] [CrossRef]
- Trilleras, J.E.; Velasquez, K.J.; Pacheco, D.; Quiroga, J.; Ortíz, A. Microwave-Assisted synthesis under solvent-free conditions of (E)-2-(benzo[d]thiazol-2-yl)-3-arylacrylonitriles. J. Braz. Chem. Soc. 2011, 22, 2396–2402. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Anichina, K.K.; Vuchev, D.I.; Tsenov, J.A.; Kondeva, M.S.; Micheva, M.K. Synthesis and antitrichinellosis activity of some 2-substituted-[1,3]thiazolo[3,2-a]benzimidazol-3(2H)-ones. Bioorg. Med. Chem. 2005, 13, 5550–5559. [Google Scholar] [CrossRef]
- Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Synthesis and structure-activity relationships of new antimicrobial active multisubstituted benzazole derivatives. Eur. J. Med. Chem. 2004, 39, 291–298. [Google Scholar] [CrossRef]
- Carta, A.; Palomba, M.; Boatto, G.; Busonera, B.; Murreddu, M.; Loddo, R. Synthesis and antiproliferative activity of 3-aryl-2-[1H(2H)-benzotriazol-1(2)-yl]acrylonitriles variously substituted: Part 4. Il Farmaco 2004, 59, 637–644. [Google Scholar] [CrossRef]
- Quiroga, J.; Cobo, D.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J.; Vásquez, Y.; Gupta, M.; Derita, M.; Zacchino, S. Synthesis and evaluation of novel E-2-(2-thienyl)- and Z-2-(3-thienyl)-3-arylacrylonitriles as antifungal and anticancer agents. Arch. Pharm. (Weinheim) 2007, 340, 603–606. [Google Scholar]
- Hranjec, M.; Pavlović, G.; Marjanović, M.; Kralj, M.; Karminski-Zamola, G. Benzimidazole derivatives related to 2,3-acrylonitriles, benzimidazo[1,2-a]quinolines and fluorenes: Synthesis, antitumor evaluation in vitro and crystal structure determination. Eur. J. Med. Chem. 2010, 45, 2405–2417. [Google Scholar] [CrossRef]
- Refaat, H.M. Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur. J. Med. Chem. 2010, 45, 2949–2956. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Ismael, M.; Del Carpio, C.A.; Tsuboi, H.; Koyama, M.; Endou, A.; Kubo, M.; Broclawik, E.; Miyamoto, A. Three-dimensional quantitative structure-activity relationship (3 D-QSAR) and docking studies on (benzothiazole-2-yl) acetonitrile derivatives as c-Jun N-terminal kinase-3 (JNK3) inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 5917–5925. [Google Scholar] [CrossRef]
- Saczewski, F.; Stencel, A.; Bieńczak, A.M.; Langowska, K.A.; Michaelis, M.; Werel, W.; Hałasa, R.; Reszka, P.; Bednarski, P.J. Structure-activity relationships of novel heteroaryl-acrylonitriles as cytotoxic and antibacterial agents. Eur. J. Med. Chem. 2008, 43, 1847–1857. [Google Scholar] [CrossRef]
- Parmar, V.S.; Kumar, A.; Prasad, A.K.; Singh, S.K.; Kumar, N.; Mukherjee, S.; Raj, H.G.; Goel, S.; Errington, W.; Puar, M.S. Synthesis of E- and Z-Pyrazolylacrylonitriles and their evaluation as novel antioxidants. Bioorg. Med. Chem. 1999, 7, 1425–1436. [Google Scholar] [CrossRef]
- Gwaram, N.S.; Ali, H.M.; Abdulla, M.A.; Buckle, M.J.C.; Sukumaran, S.D.; Chung, L.Y.; Othman, R.; Alhadi, A.A.; Yehye, W.A.; Hadi, A.H.A.; et al. ynthesis, characterization, X-ray crystallography, acetyl cholinesterase inhibition and antioxidant activities of some novel ketone derivatives of gallic hydrazide-derived Schiff base. Molecules 2012, 17, 2408–2427. [Google Scholar] [CrossRef]
- Heng, S.; Tieu, W.; Hautmann, S.; Kuan, K.; Pedersen, D.S.; Pietsch, M.; Gütschow, M.; Abell, A.D. New cholesterol esterase inhibitors based on rhodanine and thiazolidinedione scaffolds. Bioorg. Med. Chem 2011, 19, 7453–7463. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Wen, H.; Zhou, Y.; Lin, C.; Ge, H.; Ma, L.; Wang, Z.; Peng, W.; Song, H. Methyl 2-(2-(4-formylphenoxy)acetamido)-2-substituted acetate derivatives: A new class of acetylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 2123–2125. [Google Scholar]
- Sheng, R.; Lin, X.; Li, J.; Jiang, Y.; Shang, Z.; Hu, Y. Design, synthesis, and evaluation of 2-phenoxy-indan-1-one derivatives as acetylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 3834–3837. [Google Scholar] [CrossRef]
- SigmaPlot, version 10.0; The soft was used for graphic edition, SPS Software Inc.: Chicago, IL, USA, 2000.
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Maestro, version 9.0; A stand-alone software tool to configure and incorporate a range of pre-written firmware modules into your applications, Schrödinger LLC: New York, NY, USA, 2007.
- Muñoz, C.; Adasme, F.; Alzate-Morales, J.H.; Vergara-Jaque, A.; Kniess, T.; Caballero, J. Study of differences in the VEGFR2 inhibitory activities between semaxanib and SU5205 using 3D-QSAR, docking, and molecular dynamics simulation. J. Mol. Graphics Modell. 2012, 32, 39–48. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aided Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Torre, P.D.l.; Saavedra, L.A.; Caballero, J.; Quiroga, J.; Alzate-Morales, J.H.; Cabrera, M.G.; Trilleras, J. A Novel Class of Selective Acetylcholinesterase Inhibitors: Synthesis and Evaluation of (E)-2-(Benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles. Molecules 2012, 17, 12072-12085. https://doi.org/10.3390/molecules171012072
Torre PDl, Saavedra LA, Caballero J, Quiroga J, Alzate-Morales JH, Cabrera MG, Trilleras J. A Novel Class of Selective Acetylcholinesterase Inhibitors: Synthesis and Evaluation of (E)-2-(Benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles. Molecules. 2012; 17(10):12072-12085. https://doi.org/10.3390/molecules171012072
Chicago/Turabian StyleTorre, Pedro De la, Luis Astudillo Saavedra, Julio Caballero, Jairo Quiroga, Jans H. Alzate-Morales, Margarita Gutiérrez Cabrera, and Jorge Trilleras. 2012. "A Novel Class of Selective Acetylcholinesterase Inhibitors: Synthesis and Evaluation of (E)-2-(Benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles" Molecules 17, no. 10: 12072-12085. https://doi.org/10.3390/molecules171012072
APA StyleTorre, P. D. l., Saavedra, L. A., Caballero, J., Quiroga, J., Alzate-Morales, J. H., Cabrera, M. G., & Trilleras, J. (2012). A Novel Class of Selective Acetylcholinesterase Inhibitors: Synthesis and Evaluation of (E)-2-(Benzo[d]thiazol-2-yl)-3-heteroarylacrylonitriles. Molecules, 17(10), 12072-12085. https://doi.org/10.3390/molecules171012072






