The Binding Affinity and Molecular Basis of the Structure-Binding Relationship between Urinary Tamm-Horsfall Glycoprotein and Tumor Necrosis Factor-α
Abstract
:1. Introduction
2. Results and Discussion
2.1. Non-Specific THP Binding to Serum Proteins and Proinflammatory Cytokines

2.2. Dose-Dependent Binding Between THP and TNF-α
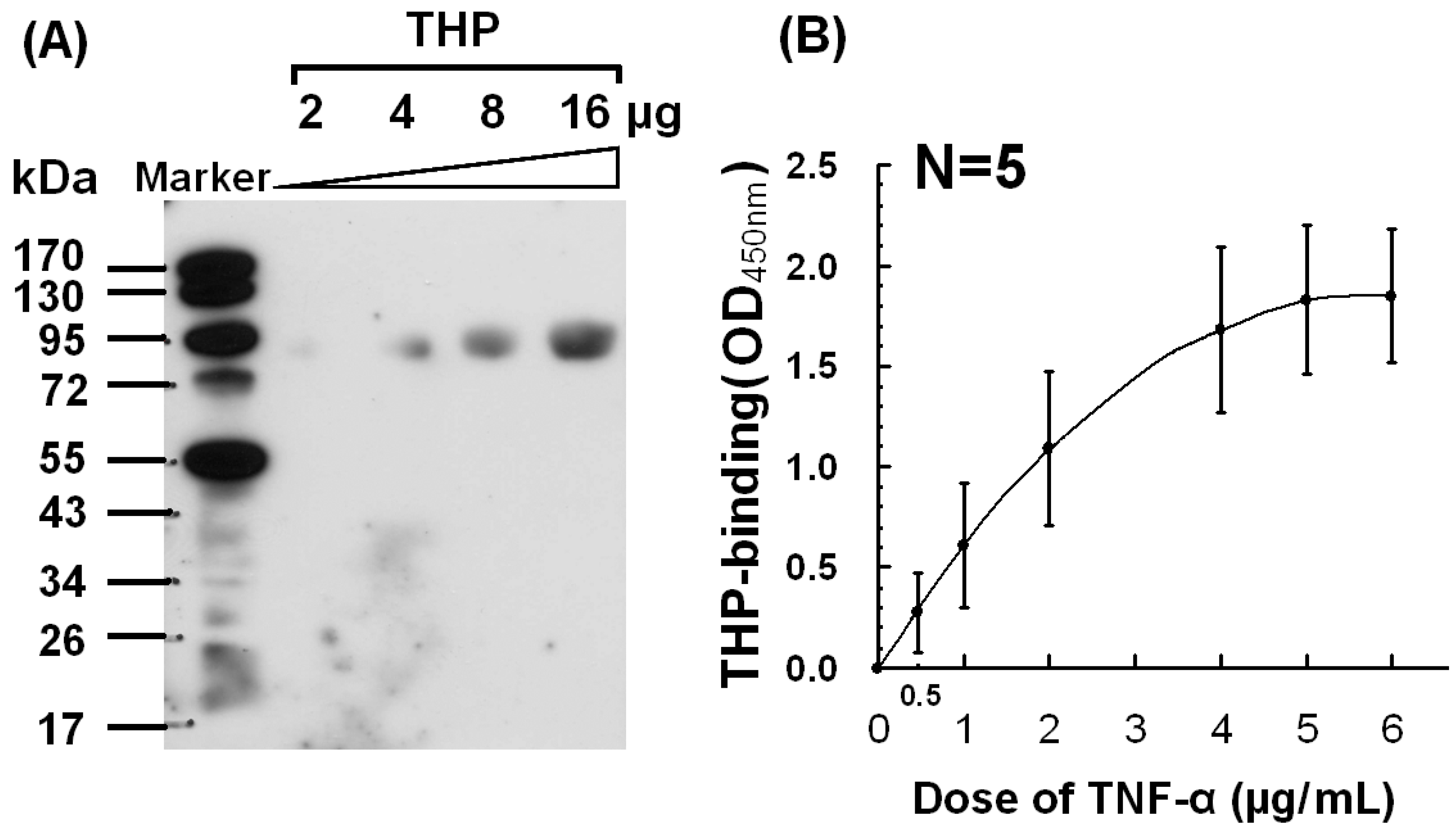
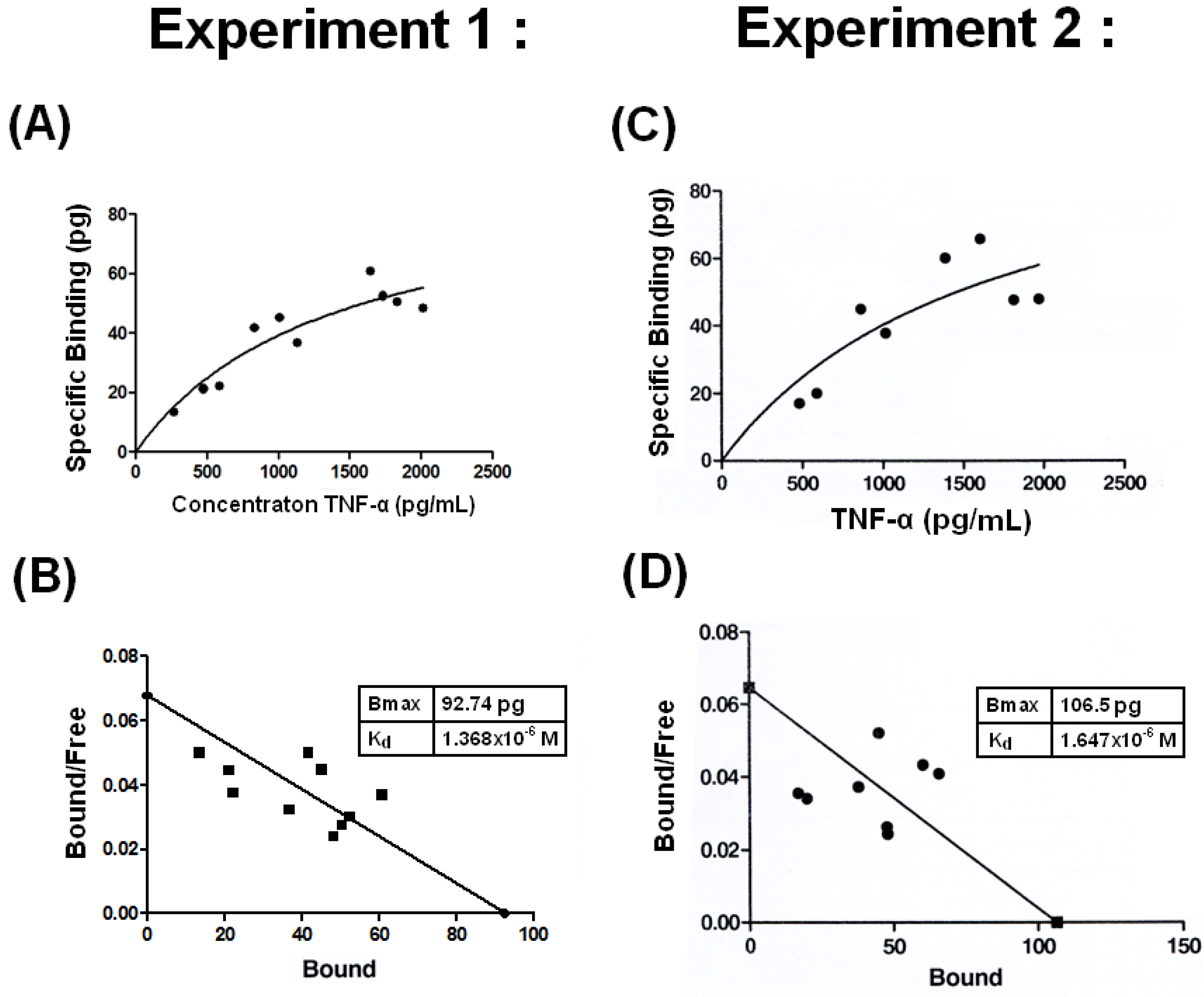
2.3. Low Binding Affinity with Kd =1.4 −1.7 × 10−6 M between THP and TNF-α
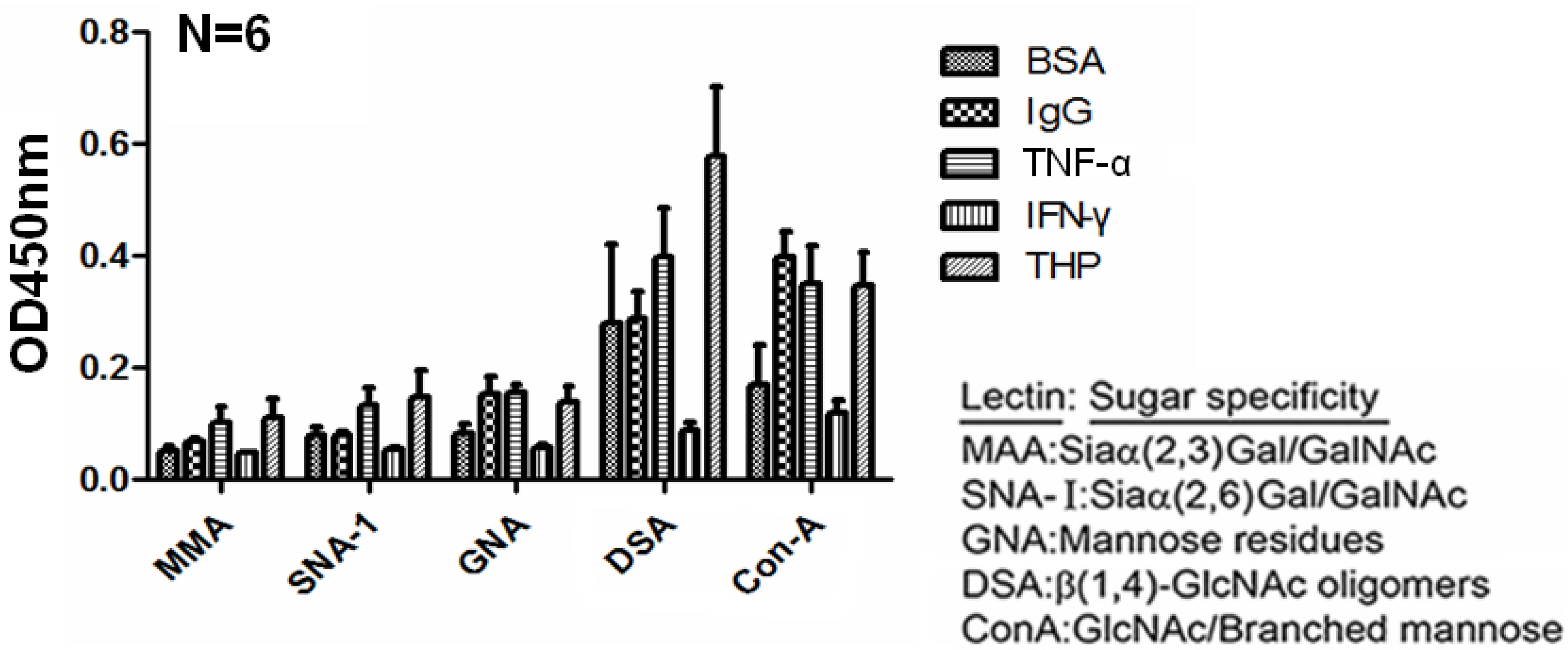
2.4. Carbohydrate Compositions of THP and THP-Binding Proteins
2.5. β-galactosidase-digested THP Enhanced THP-TNF-α Binding

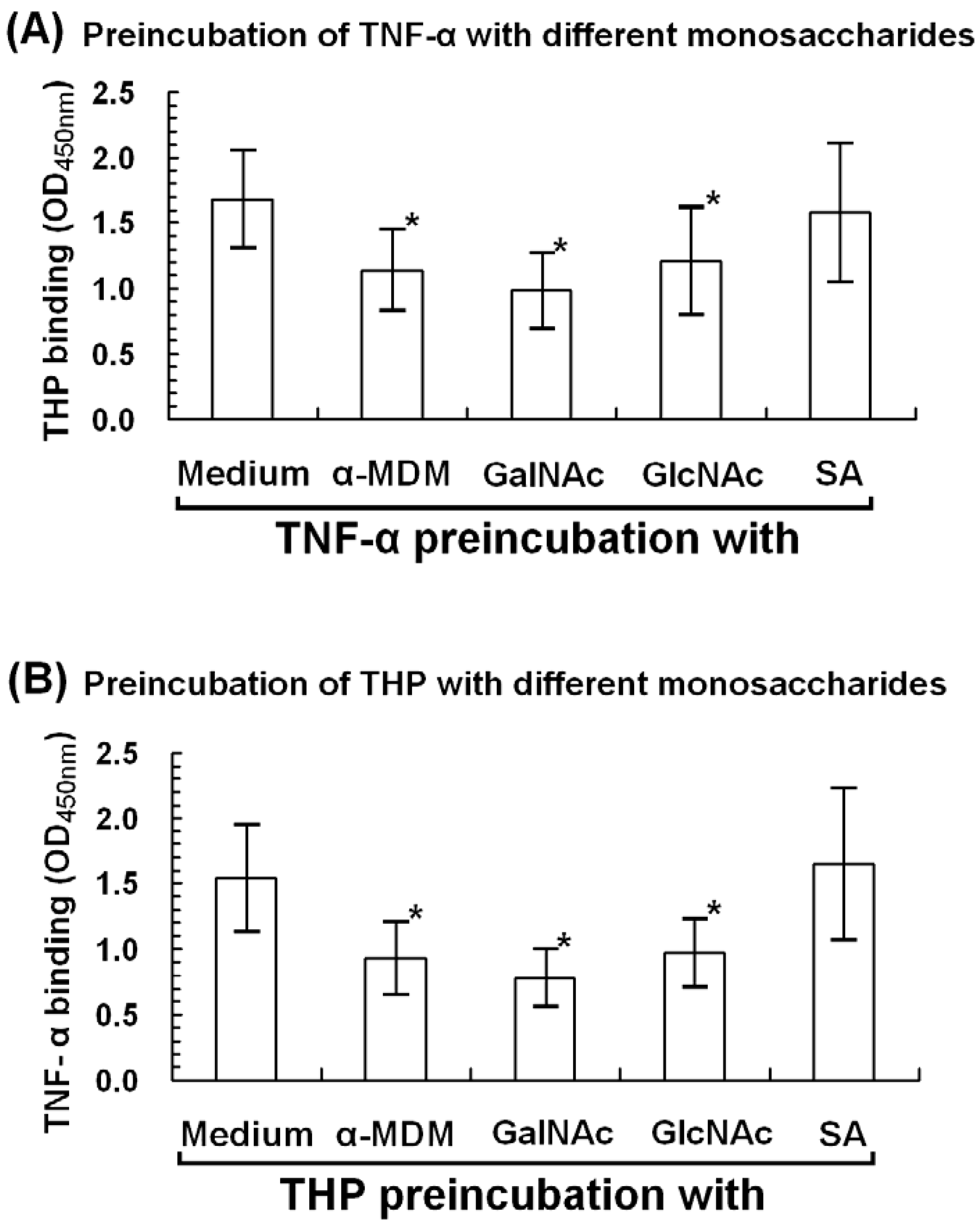
2.6. α-Methyl-D-mannoside (α-MDM), N-acetyl-galactosamine, and N-acetyl-glucosamine Mediate THP-TNF-α Binding
3. Experimental
3.1. Reagents and Antibodies
3.2. Purification of THP from Normal Human Urine
3.3. THP Digestion by Carbohydrate-, Glycoprotein-, or Protein-Specific Enzymes
3.4. Binding Activity between Intact or Enzyme-Digested THP Products and TNF-α
3.4.1. Western Blot
3.4.2. ELISA
3.5. Scatchard Plot Analysis and Kd Calculations
3.6. Monosaccharide Inhibition of THP-TNF-α Binding
3.6.1. Microwell-Coated TNF-α Was Preincubated with Different Monosaccharides
3.6.2. Microwell-Coated THP Was Preincubated with Different Monosaccharides
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Hoyer, J.K.; Seiler, M.W. Pathophysiology of Tamm-Horsfall protein. Kidney Int. 1979, 16, 279–289. [Google Scholar] [CrossRef]
- Sikri, K.L.; Foster, C.L.; Bloomfield, F.J.; Marshall, R.D. Localization by immunofluorescence and by light- and electron-microscopic immunoperoxidase techniques of Tamm-Horsfall glycoprotein in adult hamster kidney. Biochem. J. 1979, 181, 525–532. [Google Scholar]
- Tamm, I.; Horsfall, F.L. A mucoprotein derived from human urine reacts with influenza, mumps, and Newcastle viruses. Proc. Soc. Exp. Biol. Med. 1950, 74, 104–114. [Google Scholar]
- Pak, J.; Pu, Y.; Zhong, Z.-T.; Hasty, D.L.; Wu, X.-R. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli binding to uroplakin 1a and 1b receptors. J. Biol. Chem. 2001, 276, 9924–9930. [Google Scholar] [CrossRef]
- Fletcher, A.P.; Neuberger, A.; Ratcliffee, W.A. Tamm-Horsfall urinary glycoprotein: The Chemical Composition. Biochem. J. 1970, 120, 417–424. [Google Scholar]
- Stevenson, F.K.; Kent, P.W. Subunits of Tamm-Horsfall glycoprotein. Biochem. J. 1970, 116, 791–796. [Google Scholar]
- Parsons, C.L.; Rajasekaran, M.; Arsanjani, A.H.; Chenoweth, M.; Stein, P. Role of sialic acid in urinary cytoprotective activity of Tamm-Horsfall protein. Urology 2007, 69, 577–581. [Google Scholar] [CrossRef]
- Rhodes, D.C. Binding of Tamm-Horsfall protein to complement 1q measured by ELISA and resonant mirror biosensor techniques under various ionic-strength conditions. Immunol. Cell Biol. 2000, 78, 474–482. [Google Scholar] [CrossRef]
- Rhodes, D.C. Binding of Tamm-Horsfall protein to complement 1q and complement 1, including influence of hydrogen-ion concentration. Immunol. Cell Biol. 2002, 80, 558–566. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Sanders, P.W. Localization of a single-binding site for immunoglobulin-light chains on human Tamm-Horsfall glycoprotein. J. Clin. Invest. 1997, 99, 732–736. [Google Scholar] [CrossRef]
- Wu, T.-H.; Hsieh, S.-C.; Tsai, C.-Y.; Lee, Y.-F.; Tsai, C.-Y.; Yu, C.-L. Intact protein core structure is essential for protein-binding, mononuclear cell proliferating, and neutrophil phagocytosis-enhancing activities of normal human Tamm-Horsfall glycoprotein. Int. Immunopharmacol. 2008, 8, 90–99. [Google Scholar] [CrossRef]
- Muchmore, A.V.; Decker, J.M. Evidence that recombinant IL-1α exhibits lectin-like specificity and binds to homogeneous uromodulin via N-linked oligosaccharide. J. Immunol. 1987, 138, 2541–2546. [Google Scholar]
- Sherblom, A.P.; Sathyamoorthy, N.; Decker, J.M.; Muchmore, A.V. IL-2, a lectin with specificity for high mannose glycopeptides. J. Immunol. 1989, 143, 939–944. [Google Scholar]
- Sherblom, A.P.; Decker, J.M.; Muchmore, A.V. The lectin-like interaction between recombinant tumor necrosis factor and uromodulin. J. Biol. Chem. 1988, 263, 5418–5424. [Google Scholar]
- Thomas, D.B.; Davies, M.; Peters, J.R.; Williams, J.D. Tamm-Horsfall protein binds to a single class of carbohydrate specific receptor on human neutrophils. Kidney Int. 1993, 44, 423–429. [Google Scholar] [CrossRef]
- Yu, C.-L.; Tsai, C.-Y.; Sun, K.-H.; Hsieh, S.-C.; Tsai, Y.-Y.; Tsai, S.-T.; Yu, H.-S.; Han, S.-H. Tamm-Horsfall glycoprotein (THG) is a binder for surface membrane proteins on blood cells and glomerular mesangial cells. Immunopharmacology 1997, 35, 237–245. [Google Scholar] [CrossRef]
- Siao, S.-C.; Li, K.-J.; Hsieh, S.-C.; Wu, C.-H.; Lu, M.-C.; Tsai, C.-Y.; Yu, C.-L. Tamm-Horsfall glycoprotein enhances PMN phagocytosis by binding to cell surface-expressed lactoferrin and cathepsin G that activates MAP kinase pathway. Molecules 2011, 16, 2119–2134. [Google Scholar] [CrossRef]
- Su, S.-J.; Chang, K.-L.; Lin, T.-M.; Huang, Y.-H.; Yeh, T.-M. Uromodulin and Tamm-Horsfall protein induce human monocytes to secrete TNF and express tissue factor. J. Immunol. 1997, 158, 3449–3456. [Google Scholar]
- Yu, C.-L.; Lin, W.-M.; Liao, T.-S.; Tsai, C.-Y.; Sun, K.-H.; Chen, K.-H. Tamm-Horsfall glycoprotein (THG) purified from normal human pregnancy urine increases phagocytosis complement receptor expression and arachidonic acid metabolism of polymorphonuclear neutrophils. Immunopharmacology 1992, 24, 181–190. [Google Scholar] [CrossRef]
- Serafini-Cessi, F.; Dall’Olio, F.; Malagolini, N. High mannose oligosaccharides from human Tamm-Horsfall glycoprotein. Biosci. Rep. 1984, 4, 269–274. [Google Scholar] [CrossRef]
- Van Rooijen, J.J.M.; Voskamp, A.F.; Jamerling, J.P.; Vliegenthart, J.F.G. Glycosylation sites and site-specific glycosylation in human Tamm-Horsfall glycoprotein. Glycobiology 1999, 9, 21–30. [Google Scholar] [CrossRef]
- Hard, K.; van Zedelhoof, G.; Moonen, P.; Kamerling, J.P.; Vliegenthart, J.F.G. The Asn-linked carbohydrate chains of human Tamm-Horsfall glycoprotein of one male. Eur. J. Biochem. 1992, 209, 895–915. [Google Scholar] [CrossRef]
- Firon, N.; Ofek, I.; Sharon, N. Carbohydrate binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect. Immun. 1984, 43, 1088–1090. [Google Scholar]
- Easton, R.L.; Patankar, M.S.; Clark, G.F.; Morris, H.R.; Dell, A. Pregnancy associated changes in the glycosylation of Tamm-Horsfall glycoprotein; Expression of sialyl Lewis* sequences on core 2 type O-glycans derived from uromodulin. J. Biol. Chem. 2000, 275, 21928–21938. [Google Scholar]
- Isomaki, P.; Punnonen, J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann. Med. 1997, 29, 499–507. [Google Scholar] [CrossRef]
- Koenders, M.I.; Joosten, L.A.; van den Berg, W.B. Potential new targets in arthritis therapy: Interleukin (IL)-17 and its relation to tumor necrosis factor and IL-1 in experimental arthritis. Ann. Rheum. Dis. 2006, 65, iii29–iii33. [Google Scholar] [CrossRef]
- Hession, C.; Decker, J.M.; Sherblom, A.P.; Kumar, S.; Yue, C.C.; Mattaliano, R.J.; Tizard, R.; Kawashima, E.; Schmeissner, U.; Heletky, S.; et al. Uromudulin (Tamm-Horsfall glycoprotein): A renal ligand for lymphokines. Science 1987, 237, 1479–1484. [Google Scholar]
- Kievit, W.; Fransen, J.; Oerlemans, A.J.; Kuper, H.H.; van den Laai, M.A.; de Rooij, D.J.; de Gendt, C.M.; Roonday, K.H.; Jansen, T.L.; van Oijen, P.C.; et al. The efficacy of anti-TNF in rheumatoid arthritis, a comparison between randomized controlled trials and clinical practice. Ann. Rheum. Dis. 2007, 66, 1473–1478. [Google Scholar] [CrossRef]
- Aaltonen, K.J.; Virkki, L.M.; Malmivaara, A.; Konttinen, Y.T.; Nordstrom, D.C.; Blom, M. Systemic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One 2012, 7, e30275. [Google Scholar]
- Moonen, P.; Gaffiner, R.; Wingfield, P. Native cytokines do not bind to uromodulin (Tamm-Horsfall glycoprotein). FEBS Lett. 1988, 226, 314–318. [Google Scholar] [CrossRef]
- Katanick, J.; Shama, A.; DeNardin, E. Interleukin-8, neutrophil-activating peptide 2, and GRO-α bind to and elicit cell activation via specific and different amino acid residues and CXCR2. Cytokine 2000, 12, 1480–1488. [Google Scholar] [CrossRef]
- Rhodes, D.C.; Hinsman, E.J.; Rhodes, J.A. Tamm-Horsfall glycoprotein binds IgG with high affinity. Kidney Int. 1993, 44, 1014–1021. [Google Scholar] [CrossRef]
- Muchmore, A.V.; Decker, J.M.; Shaw, A.; Wingfield, P. Evidence that high mannose glycopeptides are able to functionally interact with recombinant tumor necrosis factor and recombinant interleukin 1. Cancer Res. 1990, 50, 6285–6290. [Google Scholar]
- Lucas, R.; Magez, S.; De Leys, R.; Fransen, L.; Scheerlinck, J.P.; Rampelberg, M.; Sabion, E.; De Baetselier, P. Mapping the lectin-like activity of tumor necrosis factor. Science 1994, 263, 814–817. [Google Scholar]
- Huang, Z.Q.; Sanders, P.W. Biochemical interaction between Tamm-Horsfall glycoprotein and Ig light chains in the pathogenesis of cast nephropathy. Lab. Invest. 1995, 73, 810–817. [Google Scholar]
- Munson, P.J. A user’s Guide to Ligand: Data Analysis and Curve-Fitting for Ligand Binding Experiments; National Institutes of Health: Bethesda, MD, USA, 1990. [Google Scholar]
- Sample Availability: Normal human urinary THP is available from the authors .
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, C.-H.; Li, K.-J.; Siao, S.-C.; Chen, Y.-H.; Wu, T.-H.; Tsai, C.-Y.; Yu, C.-L. The Binding Affinity and Molecular Basis of the Structure-Binding Relationship between Urinary Tamm-Horsfall Glycoprotein and Tumor Necrosis Factor-α. Molecules 2012, 17, 11978-11989. https://doi.org/10.3390/molecules171011978
Wu C-H, Li K-J, Siao S-C, Chen Y-H, Wu T-H, Tsai C-Y, Yu C-L. The Binding Affinity and Molecular Basis of the Structure-Binding Relationship between Urinary Tamm-Horsfall Glycoprotein and Tumor Necrosis Factor-α. Molecules. 2012; 17(10):11978-11989. https://doi.org/10.3390/molecules171011978
Chicago/Turabian StyleWu, Cheng-Han, Ko-Jen Li, Sue-Cien Siao, Yu-Hsuan Chen, Tsai-Hung Wu, Chang-Youh Tsai, and Chia-Li Yu. 2012. "The Binding Affinity and Molecular Basis of the Structure-Binding Relationship between Urinary Tamm-Horsfall Glycoprotein and Tumor Necrosis Factor-α" Molecules 17, no. 10: 11978-11989. https://doi.org/10.3390/molecules171011978
APA StyleWu, C.-H., Li, K.-J., Siao, S.-C., Chen, Y.-H., Wu, T.-H., Tsai, C.-Y., & Yu, C.-L. (2012). The Binding Affinity and Molecular Basis of the Structure-Binding Relationship between Urinary Tamm-Horsfall Glycoprotein and Tumor Necrosis Factor-α. Molecules, 17(10), 11978-11989. https://doi.org/10.3390/molecules171011978




