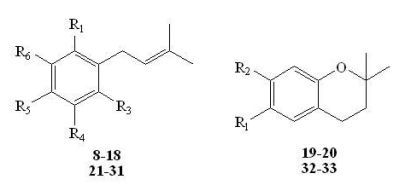
3.2. General Procedure for Preparation of Prenylated Phenols
A solution of phenol (4.5 mmol) and 3-methyl-2-buten-1-ol (7, 9.0 mmol) was placed in a round bottom flask and dissolved in dry 1:1 ethyl ether-dichloromethane (25 mL). Under nitrogen gas and with vigorous stirring, a solution of BF3 etherate (0.9 mmol) in dry 1:1 ethyl ether-dichloromethane (10 mL) was added dropwise to the solution cooled to 0–5 °C. Then the reaction mixture was allowed to warm up to room temperature and the stirring continued. After 48 h, milled ice was added to the reaction mixture and it was extracted with methylene chloride. Then, the organic layer was separated and a new extraction with ethyl acetate was done. The organic solutions obtained after extractions were mixed and dried over anhydrous sodium sulphate and filtered, the solvent was evaporated under reduced pressure. After, the mixture was subjected to silica gel flash column chromatography (ethyl acetate, petroleum ether) to obtain pure products.
3.2.1. 1,4-Dihydroxy-2-methoxy-5-(3-methyl-2-buten-1-yl) Benzene (8)
Compound 8 was obtained from 2-methoxy-1,4-dihydroxybenzene (1) as described above. The crude mixture was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford the title compound as an orange solid (126 mg, 24%); mp: 102–103 °C; IR (solution): νmax 3210 (OH), 1621 (C=C aromatic), 1434, 1190; 1H-NMR (CDCl3): 6.67 (s, 1H, ArH-6); 6.43 (s, 1H, ArH-3); 5.28 (br. t, J = 7.2 Hz, 1H, CCHCH2); 5.13 (s, 1H, ArC-1-OH) 4.79 (s, 1H, ArC-4-OH); 3.43 (s, 3H, ArC-2-OCH3); 3.25 (d, J = 7.2 Hz, 2H, C=CHCH2); 1.77 [s, 6H, CHC(CH3)2]; 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH2-); 25.7 (CH3CH3C=CH2-); 29.3 (CCHCH2-); 56.1 (CH3O-); 100.3 (ArCH-3); 115.3 (ArCH-6); 118.5 (ArC-5); 121.9 (C=CHCH2); 134.8 (C=CHCH2); 139.3 (ArC-1); 145.4 (ArC-2); 147.5 (ArC-4).
3.2.2. 1,3-Dihydroxy-4-(3-methyl-2-buten-1-yl) Benzene (9)
Compound 9 was obtained from resorcinol (2) as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 9 as an orange semi-solid (186.4 mg, 23%); IR (solution): νmax 3362 (OH), 2968 (alkane C-H), 2912 (C-H alkane), 1604 (C=C aromatic), 1518, 1451, 1157; 1H-NMR (CDCl3): 6.92 (d, 1H, J = 7.8 Hz, ArH-5); 6.37 (d, 1H, J = 8.1 Hz, ArH-6); 6.36 (s, 1H, ArH-2); 5.29 (br. t, J = 6.7 Hz, 1H, CCHCH2); 3.26 (d, J = 7.0 Hz, 2H, C=CHCH2); 1.75 (s, 3H, CHCCH3CH3); 1.74 (s, 3H, CHCCH3CH3). 13C-NMR (CDCl3): 17.7 (CH3CH3C=CH2-); 25.7 (CH3CH3C=CH2-); 28.7 [(CH3)2CCHCH2-]; 103.3 (ArC-2); 107.7 (ArC-6); 119.6 (ArC-4); 122.2 [(CH3)2C=CHCH2]; 130.5 (ArC-5); 134.2 [(CH3)2C=CHCH2]; 154.6 (ArC-1); 154.7 (ArC-3).
3.2.3. 1,4-Dihydroxy-2-(3-methyl-2-buten-1-yl) Benzene (10)
Compound
10 was obtained from hydroquinone (
3) as described above. The crude mixture was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford compound
10 as colorless needles (165.8 mg, 21%); mp: 102–104 °C, lit. [
19], 100–101 °C; IR (solution): ν
max 3228 (OH), 1654 (C=C Aromatic), 1560, 1452, 1194;
1H-NMR (CDCl
3): 6.68 (d, 1H,
J = 8.5 Hz, Ar
H-6); 6.61 (d, 1H,
J = 3.0 Hz, Ar
H-3); 6.58 (dd,
J = 8.4 and 3.0 Hz, 1H, Ar
H-5); 5.29 (br. t,
J = 7.3 Hz, 1H, C=C
HCH
2); 3.29 (d,
J = 7.2 Hz, 2H, C=CHC
H2); 1.77 [s, 6H, CHC(C
H3)
2];
13C-NMR (CDCl
3): 17.8 (
CH
3CH
3C=CH-); 25.8 (CH
3CH
3C=CH-); 29.7 [(CH
3)
2CCH
CH
2-]; 113.7 (Ar
C-5); 116.4 (Ar
C-6); 116.6 (Ar
C-3); 121.4 ((CH
3)
2C=
CHCH
2); 128.2 (Ar
C-2); 134.9 [(CH
3)
2C=CHCH
2]; 148.1 (Ar
C-1); 149.3 (Ar
C-4).
3.2.4. 1,3-Dihydroxy-5-methyl-4-(3-methyl-2-buten-1-yl) Benzene (11)
Compound 11 was obtained from orcinol (4) as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 11 as a reddish semi-solid (215.4 mg, 29%); IR (solution): νmax 3218 (OH), 2964 (C-H alkane), 2923 (C-H alkane), 1610 (C=C aromatic), 1474, 1318, 1142; 1H-NMR (CDCl3): 6.26 (d, 1H, J = 2.0 Hz, ArH-2); 6.21 (d, 1H, J = 2.1 Hz, ArH-6); 5.14 (m, 2H, OH, C=CHCH2); 4.65 (s, 1H, OH) 3.28 (d, J = 6.8 Hz, 2H, C=CHCH2); 2.23 (s, 3H, Ar-CH3); 1.80 (s, 3H, CH3CH3C=CH2-); 1.73 (s, 3H, CH3CH3C=CH2-); 13C-NMR (CDCl3): 17.9 (CH3CH3C=CH-); 20.1 (CH3CH3C=CH-); 25.2 [(CH3)2CCHCH2-]; 25.7 (Ar-CH3-5); 101.0 (ArCH-2); 109.7 (ArCH-6) 117.9 (ArC-4); 122.1 [(CH3)2C=CHCH2]; 133.9 [(CH3)2C=CHCH2]; 138.5 (ArC-5); 154.2 (ArC-1); 155.3 (ArC-3).
3.2.5. 1,2-Dihydroxy-4-(3-methyl-2-buten-1-yl) Benzene (12)
Compound 12 was obtained from pyrocatechol (5) as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 12 as a colorless solid (201.9 mg, 25%); mp: 58–61 °C; IR (solution): νmax 3364 (OH), 2924 (C-H alkane), 2853 (C-H alkane), 1603 (C=C aromatic), 1518, 1452, 1376, 1280; 1H-NMR (CDCl3): 6.77 (d, 1H, J = 8.1 Hz, ArH-6); 6.70 (d, 1H, J = 1.3 Hz, ArH-3); 6.61 (dd, 1H, J = 7.4 Hz and 1.2 Hz, ArH-5); 5.45 (s, 1H, OH) 5.37 (s, 1H, OH); 5.28 (br. t, J = 7.3 Hz, 1H, CCHCH2); 3.22 (d, J = 7.8 Hz, 2H, C=CHCH2); 1.74 (s, 3H, CHCCH3CH3); 1.70 (s, 3H, CHCCH3CH3); 13C-NMR (CDCl3): 17.7 (CH3CH3C=CH2-); 25.7 (CH3CH3C=CH2-); 33.5 [(CH3)2CCHCH2-]; 115.4 (ArC-3); 115.5 (ArC-5); 120.7 (ArC-5); 123.3 [(CH3)2C=CHCH2]; 132.4 [(CH3)2C=CHCH2]; 135.1 (ArC-4); 141.3 (ArC-1); 143.4 (ArC-2).
3.2.6. 1,3,5-Trihydroxy-2-(3-methyl-2-buten-1-yl) Benzene (13)
Compound 13 was obtained from phloroglucinol (6) as described above. The crude mixture was purified using petroleum ether-ethyl acetate (45:55) as the mobile phase to afford compound 13 as a reddish semi-solid (117.3 mg, 15%); IR (KBr): νmax 3391 (OH), 2974 (C-H alkane), 2926 (C-H alkane), 1616 (C=C aromatic), 1517, 1465, 1375, 1284, 1230, 1144; 1H-NMR [(CD3)2CO]: 7.95 (s, 2H, Ar-1,3-OH) 5.93 (s, 2H, ArH-4,6); 5.24 (br. t, 1H, J = 7.1 Hz, C=CHCH2); 3.22 (d, J = 7.1 Hz, 2H, C=CHCH2); 1.71 (s, 3H, CH3CH3C=CH2-); 1.60 (s, 3H, CH3CH3C=CH2-); 13C-NMR [(CD3)2CO]: 17.5 (CH3CH3C=CH-); 22.2 [(CH3)2CCHCH2-]; 25.5 (CH3CH3C=CH-); 95.0 (ArCH-4,6); 106.9 (ArC-2); 125.0 [(CH3)2C=CHCH2-]; 129.5 [(CH3)2C=CHCH2]; 156.6 (ArC-5); 157.0 ((ArC-1,3).
3.2.7. 1,3-Dihydroxy-4,6-di(3-methyl-2-buten-1-yl) Benzene (14)
Compound 14 was a by-product from the reaction to obtain 9 and was isolated using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 14 as a reddish oil (123.7 mg, 11%); IR (solution): νmax 3419 (OH), 2969, 2914, 2857 (C-H alkanes), 1620 (C=C aromatic), 1507, 1440, 1376, 1300, 1272, 1207, 1162, 1078; 1H-NMR (CDCl3): 6.78 (s, 1H, ArH-5); 6.33 (s, 1H, ArH-2); 5.29 (br. t, J = 6.5 Hz, 2H, 2 × CCHCH2); 5.10 (s, 2H, Ar-1,3-OH) 3.26 (d, J = 7.1 Hz, 4H, 2 × C=CHCH2); 1.77 (s, 6H, 2 × CHCCH3CH3); 1.76 (s, 6H, 2 × CHCCH3CH3); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH2-); 25.8 (CH3CH3C=CH2-); 29.3 [(CH3)2CCHCH2-]; 103.7 (ArC-2); 118.7.7 (ArC-4,6); 122.4 [(CH3)2C=CHCH2]; 130.9 (ArC-5); 134.4 [(CH3)2C=CHCH2]; 153.6 (ArC-1,3).
3.2.8. 1,4-Dihydroxy-2,5-di(3-methyl-2-buten-1-yl) Benzene (15)
Compound 15 was a by-product from the reaction to obtain 10 and was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford compound 15 as a colorless semi-solid, 34 mg, 3%; IR (solution): νmax 3223 (OH), 2978, 2926 (C-H alkanes), 1431, 1373, 1242, 1186; 1H-NMR (CDCl3): 6.57 (s, 2H, ArH-3,6); 5.28 (br. t, J = 7.2 Hz, 2H, 2 × C=CHCH2); 3.26 (d, J = 7.2 Hz, 4H, 2 × C=CHCH2); 1.76 [s, 12H, 2 × CHC(CH3)2)]; 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH-); 25.8 (CH3CH3C=CH-); 29.4 [(CH3)2CCHCH2-]; 116.9 (ArC-3,6); 121.7 [(CH3)2C=CHCH2]; 125.7 (ArC-2,5); 134.6 [(CH3)2C=CHCH2]; 147.9 (ArC-1,4).
3.2.9. 1,3-Dihydroxy-5-methyl-4,6-di(3-methyl-2-buten-1-yl) Benzene (16)
Compound 16 was a by-product from the reaction to obtain 11 and was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 16 as a pale yellow semi-solid (174.3 mg, 17%); IR (solution): νmax 3421 (OH), 2969, 2914, 2857 (C-H alkanes), 1601 (C=C aromatic), 1445, 1375, 1324, 1270, 1209, 1158, 1084; 1H-NMR (CDCl3): 6.23 (s, 1H, ArH-2); 5.12 (br. t, J = 6.7 Hz, 2H, 2 × C=CHCH2); 5.02 (s, 2H, Ar-1,3-OH); 3.33 (d, J = 6.7 Hz, 4H, 2 × C=CHCH2); 2.22 (s, 3H, Ar-CH3); 1.80 (s, 6H, 2 × CH3CH3C=CH2-); 1.72 (s, 6H, 2 × CH3CH3C=CH2-). 13C-NMR (CDCl3): 15.8 (Ar-CH3-5); 17.9 (CH3CH3C=CH-); 25.6 [(CH3)2CCHCH2-]; 25.7 (CH3CH3C=CH-); 101.2 (ArCH-2); 118.6 (ArC-4,6); 122.6 [CH3)2C=CHCH2]; 133.0 [(CH3)2C=CHCH2]; 136.4 (ArC-5) 152.7 (ArC-1,3).
3.2.10. 1,2-Dihydroxy-4,5-di(3-methyl-2-buten-1-yl) Benzene (17)
Compound 17 was a by-product from the reaction to obtain 12 and was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 17 as a reddish semi-solid (198.7 mg, 18%); IR (solution): νmax 3388 (OH), 2970, 2914, 2856 (C-H alkane), 1607 (C=C aromatic), 1514, 1477, 1448, 1375, 1282; 1H-NMR (CDCl3): 6.66 (s, 2H, ArH-3,6); 5.47 (br. s, 2H, OH); 5.20 (br. t, J = 6.9 Hz, 2H, 2 × C=CHCH2); 3.19 (d, J = 7.1 Hz, 4H, 2 × C=CHCH2); 1.72 (s, 6H, 2 × CHCCH3CH3); 1.67 (s, 6H, 2 × CHCCH3CH3); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH2-); 25.7 (CH3CH3C=CH2-); 30.8 [(CH3)2CCHCH2-]; 116.1 (ArC-3,6); 123.1 [(CH3)2C=CHCH2]; 132.2 [(CH3)2C=CHCH2]; 141.3 (ArC-1,2).
3.2.11. 1,3,5-Trihydroxy-2,6-di(3-methyl-2-buten-1-yl) Benzene (18)
Compound 18 was a by-product from the reaction to obtain 13 and was purified using petroleum ether-ethyl acetate (45:55) as the mobile phase to afford compound 18 as a reddish oil (116.7 mg, 11%); IR (solution): νmax 3434 (OH), 2971, 2915, 2857 (C-H alkanes), 1623 (C=C aromatic), 1508, 1449, 1375, 1260, 1226, 1168, 1085; 1H-NMR (CDCl3): 5.95 (s, 1H, ArH-4); 5.23 (br. t, 2H, J = 6.9 Hz, 2 × C=CHCH2); 3.33 (d, J = 7.0 Hz, 4H, 2 × C=CHCH2); 1.80 (s, 6H, 2 × CH3CH3C=CH2-); 1.74 (s, 6H, 2 × CH3CH3C=CH2-); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH-); 22.3 [(CH3)2CCHCH2-]; 25.7 (CH3CH3C=CH-); 96.0 (ArCH-4); 106.2 (ArC-2,6); 122.3 [(CH3)2C=CHCH2-]; 134.9 [(CH3)2C=CHCH2]; 153.0 (ArC-3,5); 15.0 (ArC-1).
3.2.12. 7-Hydroxy-2,2-dimethyl-chroman (19)
Compound 19was a by-product from the reaction to obtain 9 and was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 19 as a pale yellow oil (123.8 mg, 15%); IR (solution): νmax 3393 (OH), 2975, 2932, 2852 (C-H alkanes), 1622 (C=C aromatic), 1594, 1508, 1461, 1369, 1296, 1226, 1149, 1119; 1H-NMR (CDCl3): 6.90 (d, 1H, J = 8.2 Hz, ArH-5); 6.38 (dd, 1H, Jo = 8.1 Hz, Jm = 2.4 Hz, ArH-6); 6.34 (d, 1H, Jm = 2.2 Hz, ArH-8); 6.20 (s, 1H, Ar-7-OH); 2.70 (t, J = 6.6 Hz, 2H, CCH2CH2); 1.79 (t, J = 6.8 Hz, 2H, CCH2CH2); 1.34 (s, 6H, CH2CH2C(CH3)2); 13C-NMR (CDCl3): 21.6 (CH2CH2C(CH3)2); 26.7 [CH2CH2C(CH3)2]; 32.9 [CH2CH2C(CH3)2]; 74.5 [CH2CH2C(CH3)2]; 103.7 (ArCH-8); 107.6 (ArCH-6); 113.0 (ArC-4a); 130.0 (ArCH-5) 154.4 (ArC-8a); 154.9 (ArC-7).
3.2.13. 6-Hydroxy-2,2-dimethyl-chroman (20)
Compound 20 was a by-product from the reaction to obtain 10 and was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford compound 20 as a reddish oil, 194.6 mg, 24%; IR (solution): νmax 3387 (OH), 2974, 2931, 2850 (C-H alkanes), 1618 (C=C aromatic), 1492, 1449, 1369, 1452, 1243, 1200; 1H-NMR (CDCl3): 6.66–6.57 (m, 3H, ArH-5,7,8); 6.13 (s, 1H, Ar-6-OH); 2.69 (t, J = 6.7 Hz, 2H, CCH2CH2); 1.76 (t, J = 6.8 Hz, 2H, CCH2CH2); 1.32 [s, 6H, CH2CH2C(CH3)2]; 13C-NMR (CDCl3): 22.5 [CH2CH2C(CH3)2]; 26.6 [CH2CH2C(CH3)2]; 32.7 [CH2CH2C(CH3)2]; 73.9 [CH2CH2C(CH3)2]; 114.5 (ArCH-5); 115.5 (ArCH-7); 117.6 (ArCH-8), 121.7 (ArC-4a); 147.5 (ArC-8a); 148.6 (ArC-6).
3.3. General Procedure for the Acetylation Reactions
To a stirred solution of prenylated phenol (1 equiv.) in methylene chloride (10 mL) was added dimethylaminopyridine (0.1 equiv.) and acetic anhydride (4 equiv.) at room temperature. After 1 h, the solvent was evapored under reduced pressure. Finally, the mixture was subjected to silica gel flash column chromatography (ethyl acetate, petroleum ether) to obtain pure products.
3.3.1. 1,4-Diacetoxy-2-metoxi-5-(3-methyl-2-buten-1-yl) Benzene (21)
Compound 21 was obtained from 8 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (60:40) as the mobile phase to afford compound 21 as a slightly orange solid (67.1 mg, 90%); MS m/z: 292 (11%), 250 (22%), 208 (98%), 153 (100%), 69 (9%); mp: 75–77 °C; IR (solution): νmax 1765 (C=O ester), 1621 (C=C aromatic), 1511, 1368, 1206; 1H-NMR (CDCl3): 6.87 (s, 1H, ArH-6); 6.65 (s, 1H, ArH-3); 5.18 (br. t, J = 7.2 Hz, 1H, CCHCH2); 3.78 (s, 3H, ArC-2-OCH3); 3.14 (d, J = 7.2 Hz, 2H, C=CHCH2); 2.29 (s, 6H, Ar-1,4-OCOCH3); 1.73 [s, 3H, CHC(CH3) (CH3)]; 1.67 [s, 3H, CHC(CH3) (CH3)]. 13C-NMR (CDCl3): 17.7 (CH3CH3C=CH2-); 20.6 (OCOCH3); 20.8 (OCOCH3); 25.6 (CH3CH3C=CH2-); 27.8 (CCHCH2-); 56.1 (CH3O-); 107.0 (CH-Ar-3); 121.3 (C=CHCH2); 123.4 (ArCH-6); 125.5 (ArC-4); 133.4 (C=CHCH2); 137.3 (ArC-1); 146.5 (ArC-5); 149.5 (ArC-2); 168.9 (OCOCH3); 169.2 (OCOCH3).
3.3.2. 1,3-Diacetoxy-4-(3-methyl-2-buten-1-yl) Benzene (22)
Compound 22 was obtained from 9 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 22 as a colorless semisolid (214.5 mg, 61%); MS m/z: 262 (2%), 203 (20%), 178 (20%), 163 (100%), 135 (7%) 107 (9%); IR (solution): νmax 2978 (C-H alkane), 1766 (C=O ester), 1609 (C=C aromatic), 1496, 1422, 1370, 1198 (C-O); 1H-NMR (CDCl3): 7.22 (d, J = 8.4 Hz, 1H, ArH-5); 6.93 (dd, J = 8.4 and 2.2 Hz, 1H, ArH-6); 6.86 (d, J = 2.2 Hz, 1H, ArH-2); 5.21 (br. t, J = 7.2, 1H, CCHCH2); 3.22 (d, J = 7.2 Hz, 2H, CCHCH2); 2.29 (s, 3H, OCOCH3); 2.26 (s, 3H, OCOCH3); 1.74 [s, 3H, CHC(CH3) (CH3)]; 1.69 [s, 3H, CHC(CH3) (CH3)]; 13C-NMR (CDCl3): 17.7 (CH3CH3C=CH2-); 20.7 (OCOCH3); 20.9 (OCOCH3); 25.6 (CH3CH3C=CH2-); 28.3 [(CH3)2CCHCH2-]; 115.8 (ArC-2); 119.0 (ArC-6); 121.3 [(CH3)2C=CHCH2]; 130.1 (ArC-5); 130.9 (ArC-4); 133.2 [(CH3)2C=CHCH2]; 148.8 (ArC-1); 148.9 (ArC-3); 168.8 (OCOCH3); 169.0 (OCOCH3).
3.3.3. 1,4-diacetoxy-2-(3-methyl-2-buten-1-yl) benzene (23)
Compound 23was obtained from 10 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford compound 23 as a colorless oil (115.2 mg, 98%); MS m/z: 262 (5%), 219 (17%), 178 (100%), 163 (18%), 123 (36%); IR (solution): νmax 2970 (C-H alkane), 2916 (C-H alkane), 1763 (C=O ester), 1616 (C=C aromatic), 1491, 1438, 1369, 1208 (C-O), 1171 (C-O); 1H-NMR (CDCl3): 7.02 (d, J = 9.5 Hz, 1H, ArH-6); 6.95 (m, 2H, ArH-3,5); 5.21 (br. t, J = 7.3 Hz, 1H, C=CHCH2); 3.22 (d, J = 7.2 Hz, 2H, C=CHCH2); 2.30 (s, 3H, OCOCH3); 2.28 (s, 3H, OCOCH3); 1.74 (s, 3H, CH3CH3C=CH-); 1.68 (s, 3H, CH3CH3C=CH-); 13C-NMR (100 MHz, CDCl3): 17.8 (CH3CH3C=CH-); 20.8 (OCOCH3); 21.1 (OCOCH3); 25.7 (CH3CH3C=CH-); 28.6 [(CH3)2C=CHCH2-]; 119.9 (ArCH-5); 120.8 [(CH3)2C=CHCH2]; 122.7 (ArCH-3) 122.9 (ArCH-6); 133.8 [(CH3)2C=CHCH2]; 134.9 (ArC-2); 146.2 (ArC-1); 148.2 (ArC-4); 169.2 (OCOCH3); 169.4 (OCOCH3).
3.3.4. 1,3-Diacetoxy-5-methyl-4-(3-methyl-2-buten-1-yl) Benzene (24)
Compound 24 was obtained from 11 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 24 as a colorless oil (133.5 mg, 63%); MS m/z: 276 (1%), 233 (37%), 192 (35%), 177 (19%), 137 (100%). IR (solution): νmax 2924 (C-H alkane), 1770 (C=O ester), 1618 (C=C aromatic), 1480, 1368, 1198 (C-O); 1H-NMR (CDCl3): 6.81 (d, 1H, J = 1.9 Hz, ArH-6); 6.21 (d, 1H, J = 2.0 Hz, ArH-2); 4.98 (br. t, J = 6.6 Hz, 1H,C=CHCH2); 3.22 (d, J = 6.6 Hz, 2H, C=CHCH2); 2.30 (s, 3H, Ar-CH3); 2.28 (s, 3H, OCOCH3); 2.26 (s, 3H, OCOCH3); 1.74 (s, 3H, CH3CH3C=CH2-); 1.68 (s, 3H, CH3CH3C=CH2-); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH-); 19.7 (Ar-CH3-5); 20.8 (OCOCH3); 21.0 (OCOCH3); 25.5 (CH3CH3C=CH-); 25.8 [(CH3)2CCHCH2-]; 113.5 (ArCH-2); 120.8 (ArCH-6); 121.3 [(CH3)2C=CHCH2]; 129.5 (ArC-4); 131.9 [(CH3)2C=CHCH2]; 139.0 (ArC-5); 148.3 (ArC-1); 149.0 (ArC-3); 169.1 (OCOCH3); 169.2 (OCOCH3).
3.3.5. 1,2-Diacetoxy-4-(3-methyl-2-buten-1-yl) Benzene (25)
Compound 25 was obtained from 12 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 25 as a colorless oil (84 mg, 92%); MS m/z: 262 (5.5%), 220 (25%), 178 (100%), 163 (45%), 145 (24%). IR (solution): νmax 2979 (C-H alkane), 2936 (C-H alkane), 1771 (C=O ester), 1607 (C=C aromatic), 1505, 1428, 1372, 1210 (C-O); 1H-NMR (CDCl3): 7.06 (m, 2H, ArH-3,5); 6.70 (s, 1H, ArH-6); 5.30 (br. t, J = 7.4 Hz, 1H, CCHCH2); 3.34 (d, J = 7.8 Hz, 2H, C=CHCH2); 2.28 (s, 3H, OCOCH3); 2.27 (s, 3H, OCOCH3); 1.75 (s, 3H, CHCCH3CH3); 1.69 (s, 3H, CHCCH3CH3); 13C-NMR (CDCl3): 17.7 (CH3CH3C=CH2-); 20.6 (OCOCH3 × 2); 25.7 (CH3CH3C=CH2-); 33.5 [(CH3)2CCHCH2-]; 122.1 [(CH3)2C=CHCH2]; 123.0 (ArC-3,6); 126.3 (ArC-5); 133.3 [(CH3)2C=CHCH2]; 139.9 (ArC-2); 140.6 (ArC-4); 141.7 (ArC-1); 168.3 (OCOCH3); 168.4 (OCOCH3).
3.3.6. 1,3,5-Triacetoxy-2-(3-methyl-2-buten-1-yl) Benzene (26)
Compound 26 was obtained from 13 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (45:55) as a mobile phase to afford compound 26 as a colorless oil (207.6 mg, 63%); MS m/z: 320 (1%), 277 (38.5%), 235 (50%), 194 (79%), 139 (100%); IR (solution): νmax 2972 (C-H alkane), 2927 (C-H alkane), 1772 (C=O ester), 1620 (C=C Aromatic), 1481, 1430, 1370, 1193 (C-O); 1H-NMR (CDCl3): 6,82 (s, 2H, ArH-4,6); 5,00 (br. t, 1H, J = 6.9 Hz, C=CHCH2); 3.15 (d, J = 6.8 Hz, 2H, C=CHCH2); 2.27 [s, 6H, ArC-1,3-(OCOCH3)2]; 2.25 (s, 3H, ArC-5-OCOCH3); 1.71 (s, 3H, CH3CH3C=CH2-); 1.66 (s, 3H, CH3CH3C=CH2-); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH-); 20.8 (‑OCOCH3); 21.0 (OCOCH3); 23.7 [(CH3)2CCHCH2-]; 25.5 (CH3CH3C=CH-); 113.8 (ArCH-4,6); 120.9 [(CH3)2C=CHCH2-]; 123.9 (ArC-2); 132.2 [(CH3)2C=CHCH2]; 148.4 (ArC-5); 149.5 (ArC-1,3); 168.6 [(-OCOCH3)3].
3.3.7. 1,3-Diacetoxy-4,6-di(3-methyl-2-buten-1-yl) Benzene (27)
Compound 27 was obtained from 14 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 27 as a colorless semisolid (101.5 mg, 43%); MS m/z: 330 (1%), 287 (30%), 245 (29%), 191 (100%), 177 (12%) 69 (50); IR (solution): νmax 2973, 2915 (C-H alkanes), 1766 (C=O ester), 1592 (C=C aromatic), 1592, 1496, 1437, 1404, 1369, 1198 (C-O); 1H-NMR (CDCl3): 7.04 (s, 1H, ArH-5); 6.78 (s, 1H, ArH-2); 5.19 (br. t, J = 7.1, 2H, 2 × CCHCH2); 3.18 (d, J = 7.2 Hz, 4H, 2 × CCHCH2); 2.27 (s, 6H, 2 × OCOCH3); 1.73 [s, 6H, 2 × CHC(CH3) (CH3)]; 1.69 [s, 6H, 2 × CHC(CH3) (CH3)]; 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH2-); 20.8 (OCOCH3); 25.6 (CH3CH3C=CH2-); 28.5 [(CH3)2CCHCH2-]; 116.3 (ArC-2); 121.6 [(CH3)2C=CHCH2]; 130.9 (ArC-5); 131.0 (ArC-4,6); 133.1 [(CH3)2C=CHCH2]; 146.9 (ArC-1,3); 169.1 (OCOCH3).
3.3.8. 1,4-Diacetoxy-2,5-di(3-methyl-2-buten-1-yl) Benzene (28)
Compound 28 was obtained from 15 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford compound 28 as a colorless semisolid (29.3 mg, 88%); MS m/z: 330 (4%), 287 (29%), 246 (100%), 190 (65%), 69 (45%); IR (solution): νmax 2968, 2910, 2857 (C-H alkanes), 1755 (C=O ester), 1498, 1560, 1440, 1366, 1221 (C-O), 1209 (C-O), 1183; 1H-NMR (CDCl3): 6.85 (s, 2H, ArH-3,6); 5.19 (br. t, J = 7.2 Hz, 2H, 2 × C=CHCH2); 3.17 (d, J = 7.1 Hz, 4H, 2 × C=CHCH2); 2.29 (s, 6H, OCOCH3) 1.73 (s, 6H, 2 × CH3CH3C=CH-) 1.66 (s, 6H, 2 × CH3CH3C=CH-); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH-); 20.9 (OCOCH3); 25.7 (CH3CH3C=CH-); 28.4 [(CH3)2CCHCH2-]; 121.1 [(CH3)2C=CHCH2]; 123.2 (ArC-3,6); 132.2 (ArC-2,5); 133.6 [(CH3)2C=CHCH2]; 146.4 (ArC-1,4); 169.4 (OCOCH3).
3.3.9. 1,3-Diacetoxy-5-methyl-4,6-di(3-methyl-2-buten-1-yl) Benzene (29)
Compound 29 was obtained from 16 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (70:30) as the mobile phase to afford compound 29 as a colorless semi-solid (48.5 mg, 62%); MS m/z: 344 (1%), 301 (17%), 285 (7%), 259 (42%), 245 (18%), 227 (31%) 205 (100%), 161 (17%), 69 (35%). IR (solution): νmax 2969, 2916 (C-H alkanes), 1768 (C=O ester), 1596 (C=C aromatic), 1447, 1368, 1288, 1222, 1197 (C-O); 1H-NMR (CDCl3): 6.67 (s, 1H, ArH-2); 4.98 (br. t, J = 6.6 Hz, 2H, 2 × C=CHCH2); 3.23 (d, J = 6.5 Hz, 4H, 2 × C=CHCH2); 2.28 (s, 6H, 2 × OCOCH3); 2.22 (s, 3H, Ar-CH3); 1.74 (s, 6H, 2 × CH3CH3C=CH2-); 1.64 (s, 6H, 2 × CH3CH3C=CH2-); 13C-NMR (CDCl3): 15.5 (Ar-CH3-5); 17.9 (CH3CH3C=CH-); 20.9 (CH3CH3C=CH-); 25.6 (OCOCH3); 26.4 [(CH3)2CCHCH2-]; 114.0 (ArCH-2); 121.7 [(CH3)2C=CHCH2]; 130.0 (ArC-4,6); 131.7 [(CH3)2C=CHCH2]; 137.9 (ArC-5); 149.0 (ArC-1,3); 169.4 (OCOCH3).
3.3.10. 1,2-Diacetoxy-4,5-di(3-methyl-2-buten-1-yl) Benzene (30)
Compound 30 was obtained from 17 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford compound 30 as a colorless oil (99 mg, 92%); MS m/z: 330 (0.6%), 288 (9%), 246 (9%), 232 (11%), 190 (100%), 175 (87%), 131 (11%), 91 (15%), 69 (23%); IR (solution): νmax 2971, 2915 (C-H alkanes), 1774 (C=O ester), 1499, 1437, 1370, 1275, 1210 (C-O). 1H-NMR (CDCl3): 6.93 (s, 2H, ArH-3,6); 5.23 (br. t, J = 7.2 Hz, 2H, 2 × CCHCH2); 3.28 (d, J = 7.2 Hz, 4H, 2 × C=CHCH2); 2.27 (s, 6H, 2 × OCOCH3); 1.75 (s, 6H, 2 × CHCCH3CH3); 1.68 (s, 6H, 2 × CHCCH3CH3); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH2-); 20.6 (OCOCH3 × 2); 25.7 (CH3CH3C=CH2-); 30.9 [(CH3)2CCHCH2-]; 121.8 [(CH3)2C=CHCH2]; 123.1 (ArCH-3,6); 133.3 [(CH3)2C=CHCH2]; 138.3 (ArC-4,5); 139.7 (ArC-1,2); 168.5 (OCOCH3).
3.3.11. 1,3,5-Triacetoxy-2,6-di(3-methyl-2-buten-1-yl) Benzene (31)
Compound 31 was obtained from 18 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (45:55) as the mobile phase to afford compound 31 as a colorless semi-solid (111.0 mg, 60%); MS m/z: 388 (0.1%), 345 (26%), 303 (24%), 287 (17%), 271 (39%), 261 (28%), 247 (62%), 205 (100%), 191 (38%), 163 (40%), 151 (40%), 69 (34%); IR (solution): νmax 2972, 2924 (C-H alkanes), 1770 (C=O ester), 1614 (C=C aromatic), 1475, 1423, 1369, 1193 (C-O); 1H-NMR (CDCl3): 6,82 (s, 1H, ArH-6); 5,00 (br. t, 2H, J = 6.7 Hz, 2 × C=CHCH2); 3.11 (s, 4H, 2 × C=CHCH2); 2.27 [s, 3H, ArC-3-(OCOCH3)2]; 2.25 (s, 6H, ArC-1,5-OCOCH3); 1.70 (s, 6H, 2 × CH3CH3C=CH2-); 1.67 (s, 6H, CH3CH3C=CH2-); 13C-NMR (CDCl3): 17.8 (CH3CH3C=CH-); 20.5 (-OCOCH3); 20.8 (-OCOCH3); 21.0 (OCOCH3); 24.4 [(CH3)2CCHCH2-]; 25.6 (CH3CH3C=CH-); 115.0 (ArCH-6); 121.2 [(CH3)2C=CHCH2-]; 124.6 (ArC-2,4); 132.0 [(CH3)2C=CHCH2]; 147.3 [(ArC-1,3,5]; 168.7 (-OCOCH3).
3.3.12. 7-Acetoxy-2,2-dimethyl-chroman (32)
Compound 32 was obtained from 19 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (90:10) as the mobile phase to afford compound 32 as a colorless semisolid (165.2 mg, 41%); MS m/z: 220 (9%), 178 (30%), 163 (14%), 123 (100%), 65 (14%); IR (solution): νmax 2976, 2933, 2854 (C-H alkane), 1765 (C=O ester), 1615 (C=C aromatic), 1589, 1499, 1464, 1427, 1307, 1208 (C-O); 1H-NMR (CDCl3): 7.04 (d, 1H, J = 8.2 Hz, ArH-5); 6.55 (dd, 1H, Jo = 8.2 Hz, Jm = 2.1 Hz, ArH-6); 6.51 (d, 1H, Jm = 2.1 Hz, ArH-8); 2.75 (t, J = 6.7 Hz, 2H, CCH2CH2); 2.27 (s, 3H, OCOCH3); 1.79 (t, J = 6.7 Hz, 2H, CCH2CH2); 1.33 [s, 6H, CH2CH2C(CH3)2]; 13C-NMR (CDCl3): 21.0 (OCOCH3); 22.0 [CH2CH2C(CH3)2]; 26.8 [CH2CH2C(CH3)2]; 32.5 [CH2CH2C(CH3)2]; 74.4 [CH2CH2C(CH3)2]; 110.3 (ArCH-8); 112.8 (ArCH-6); 118.5 (ArC-4a); 129.7 (ArCH-5) 149.6 (ArC-7); 154.6 (ArC-8a); 169.5 (OCOCH3).
3.3.13. 6-Acetoxy-2,2-dimethyl-chroman (33)
Compound 33 was obtained from 20 as described above. The crude mixture was purified using petroleum ether-ethyl acetate (80:20) as the mobile phase to afford compound 33 (210.1 mg, 76%); MS m/z: 220 (18%), 178 (100%), 149 (15%), 163 (23%), 123 (62%); IR (solution): νmax 2976, 2933, 2854 (C-H alkanes), 1765 (C=O ester), 1614 (C=C aromatic), 1589, 1499, 1464, 1426, 1370, 1209 (C-O), 1142; 1H-NMR (CDCl3): 6.78 (m, 3H, ArH-5,7,8); 2.76 (t, J = 6.8 Hz, 2H, Ar-CH2CH2); 2.26 (s, 3H, OCOCH3); 1.78 (t, J = 6.8 Hz, 2H, Ar-CH2CH2-C-O); 1.32 [s, 6H, (CH3)2C]; 13C-NMR (CDCl3): 21.1 (OCOCH3); 22.6 (Ar-CH2CH2-C-O); 26.8 ((CH3)2C); 32.5 (Ar-CH2CH2-C-O); 74.3 [(CH3)2C]; 117.7 (ArCH-8); 120.2 (ArCH-7); 121.5 (ArC-4a); 121.8 (ArCH-5); 143.2 (ArC-6); 151.7 (ArC-8a); 170.0 (OCOCH3).