A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones
Abstract
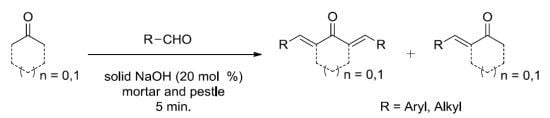
:1. Introduction
2. Results and Discussion
2.1. α,α'-bis-(Substitutedbenzylidene)cycloalkanones

| Entry | NaOH (mol%) [a] | Time (min.) | Yield [b] |
|---|---|---|---|
| 1 | 100 | 5 | 99 |
| 2 | 80 | 5 | 99 |
| 3 | 40 | 5 | 98 |
| 4 | 20 | 5 | 98 |
| 5 | 10 | 5 | 95 |
| 6 | 1 | 5 | 70 |
| Entry | Catalysts | Time | Yield [a] (%) |
|---|---|---|---|
| [a] Isolated yields which are not optimized. | |||
| 1 | NaOH (20 mmol), mortar and pestle | 5 min | 98 |
| 2 | KOH (20 mmol), mortar and pestle | 5 min | 85 |
| 3 | NaOH (20 mmol), r.t, EtOH | 24 h | 40 |
| 4 | NaOH (20 mmol), r.t, EtOH | 96 h | 60 |
| 5 | NaOH (20 mmol), r.t, EtOH | 5 d | 66 |
| 6 | NaOH (20 mmol), reflux , EtOH | 8 h | 93 |
| 7 | NaOAc (20 mmol), AcOH, 120 °C | 8 h | 81–93[74] |
| 8 | NH4OAc (4 mmol), AcOH, 120 °C | 8 h | 83–95[75] |
 | ||||
|---|---|---|---|---|
| Entry | R | n | Yield (%) | mp (°C) (lit.value) [references] |
| 3a | H | 0 | 98 | 188, (188–190) [ 74], (188–189) [38] |
| 3b | 2-Br | 0 | 96 | 165, (163–165) [ 74], (162–163) [40] |
| 3c | 4-Me | 0 | 98 | 184, (183–184) [ 40] |
| 3d | 4-OMe | 0 | 98 | 211, (210–211) [ 74], (210–211) [38] |
| 3e | H | 1 | 98 | 119, (119–120) [ 74], (117–118) [38] |
| 3f | 2-NO2 | 1 | 98 | 159, (158–159) [ 74], (158–159) [74] |
| 3g | 3-Cl | 1 | 97 | 104, (103–105) [ 74] |
| 3h | 4-Me | 1 | 98 | 168, (165–167) [ 74], (170.1) [76] |

2.2. Reaction of Acetone (5) with Benzaldehyde (2a)

| Molar ratio | Conversion(%) | Yield [a] (%) | ||
|---|---|---|---|---|
| 5 | 2a | 6 | 7 | |
| 10 mmol | 20 mmol | 100 b | 53 | 42 |
| Excess (>5 equiv.) | 10 mmol | 100 b | trace | 96 |
| 10 mmol | Excess (>3 equiv.) | 100 c | 98 | 0 |
2.3. Reaction of 1,7,7-Trimethyl[2,2,1]hexan-2-one (Camphor, 8) with Substituted-benzaldehyde (2a and 2c)

3. Experimental
3.1. General
3.2. Chemistry
3.2.1. General Procedure for the Preparation of α,α'-bis-(Substituted-benzylidene)cycloalkanones 3a–h
3.2.2. General Procedure for the Preparation of 3i, 3j and 4
3.2.3. Procedure for the Preparation of (1E,4E)-1,5-Diphenylpenta-1,4-dien-3-one (6) and (E)-4-Phenylbut-3-en-2-one (7)
3.2.4. Preparation of (E)-3-Substitutedbenzylidene-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one (9)
4. Conclusions
References and Notes
- Nielsen, A.T.; Houlihan, W.J. Organic Reactions. In The Aldol Condensation; Adams, R., Blatt, A.H., Boekelheide, V., Cairns, T.L., Cram, D.J., House, H.O., Eds.; J. Wiley & Sons: New York, NY, USA, 1968; Volume 16, pp. 1–438. [Google Scholar]
- Mukaiyama, T. Organic Reactions; Dauben, W.G., Ed.; J. Wiley & Sons: New York, NY, USA, 1982; Volume 28, pp. 203–331. [Google Scholar]
- Heathcock, C.H. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 2, pp. 133–179. [Google Scholar]
- Gennari, C. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 2, pp. 629–660. [Google Scholar]
- Mahrwald, R., Ed. ; Modern Aldol Reactions; Wiley-VCH: Weinheim, Germany, 2004; Volume 1 and 2. [Google Scholar]
- Reeves, R.L. Chemistry of Carbonyl Group; Patai, S., Ed.; Wiley-Intersciences: New York, NY, USA, 1966; pp. 580–600. [Google Scholar]
- Robinson, T.P.; Ehlers, T.; Hubbard, R.B.; Bai, X.; Arbiser, J.L.; Goldsmith, D.J.; Bowena, J.P. Design, synthesis, and biological evaluation of angiogenesis inhibitors: Aromatic enone and dienone analogues of curcumin. Bioorg. Med. Chem. Lett. 2003, 13, 115–117. [Google Scholar] [CrossRef]
- Robinson, T.P.; Hubbard, R.B.; Ehlers, T.J.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg. Med. Chem. 2005, 13, 4007–4013. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abeygunawardana, C.; Talalay, P. Chemoprotective Properties of Phenylpropenoids, Bis(benzylidene)cycloalkanones, and Related Michael Reaction Acceptors: Correlation of Potencies as Phase 2 Enzyme Inducers and Radical Scavengers. J. Med. Chem. 1998, 41, 5287–5296. [Google Scholar] [CrossRef]
- Cheng, D.; Valente, S.; Castellano, S.; Sbardella, G.; Di Santo, R.; Costi, R.; Bedford, M.T.; Mai, A. Novel 3,5-Bis(bromohydroxybenzylidene)piperidin-4-ones as Coactivator-Associated Arginine Methyltransferase 1 Inhibitors: Enzyme Selectivity and Cellular Activity. J. Med. Chem. 2011, 54, 4928–4932. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Padmanilayam, M.P.; Zello, G.A.; Nienaber, K.H.; Allen, T.M.; Santos, C.L.; De Clercq, E.; Balzarini, J.; Manavathu, E.K.; Stables, J.P. Cytotoxic analogues of 2,6-bis(arylidene)cyclohexanones. Eur. J. Med. Chem. 2003, 38, 169–177. [Google Scholar] [CrossRef]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef]
- Piantadosi, C.; Hall, I.H.; Irvine, J.L.; Carlson, G.L. Cycloalkanones. 2. Synthesis and biological activity of alpha, alpha'-dibenzylcycloalkanones. J. Med. Chem. 1973, 16, 770–795. [Google Scholar] [CrossRef]
- Ogawa, M.; Ishii, Y.; Nakano, T.; Irifune, S. Production of 2-alkylidenecycloalkanone derivative. Jpn. Kohai Tokkyo JP 1988, 63238034–A2. [Google Scholar]
- Raj, A.A.; Raghunathan, R. double 1,3-dipolar cycloaddition of 2,6-bis(arylmethylidene)-cyclohexanones: A novel entry into the synthesis of 2′,6′- bis-spiro(1-n-methyl-4-aryl-pyrrolidine)cyclohexanones. Synth. Commun. 2001, 32, 3295–3300. [Google Scholar]
- Raj, A.A.; Raghunathan, R.; Sridevi Kumari, M.R.; Raman, N. Synthesis, Antimicrobial and Antifungal Activity of a New Class of Spiro pyrrolidines. Bioorg. Med. Chem. 2003, 11, 407–419. [Google Scholar] [CrossRef]
- Gangadhara, K.K. Synthesis and characterization of photo-crosslinkable main-chain liquid-crystalline polymers containing bis(benzylidene)cycloalkanone units. Polymer 1995, 36, 1903–1910. [Google Scholar] [CrossRef]
- Deli, J.; Lorand, T.; Szabo, D.; Foldesi, A. Potential bioactive pyrimidine derivatives, part 1: 2-Amino-4-aryl-8-arylidene-3,4,5,6,7,8 hexahydroquinazolines. Pharmazie 1984, 39, 539–540. [Google Scholar]
- Leonard, N.J.; Miller, L.A.; Berry, J.W. The Synthesis of 2,7-Disubstituted Tropones via Aromatization. J. Am. Chem. Soc. 1957, 79, 1482–1485. [Google Scholar] [CrossRef]
- Ciufolini, M.A.; Byrne, N.E. The total synthesis of cystodytins. J. Am. Chem. Soc. 1991, 113, 8016–8024. [Google Scholar] [CrossRef]
- Das, U.; Kawase, M.; Sakagami, H.; Ideo, A.; Shimada, J.; Molnar, J.; Barath, Z.; Bata, Z.; Dimmock, J.R. 3-(3,4,5-Trimethoxyphenyl)-1-oxo-2-propene: A novel pharmacophore displaying potent multidrug resistance reversal and selective cytotoxicity. Bioorg. Med. Chem. 2007, 15, 3373–3380. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Hamon, N.W.; Hindmarsh, K.W.; Sellar, A.P.; Turner, W.A.; Rank, G.H.; Robertson, A.J. Evaluation of 2-benzylidenecyclohexanones and 2,6-bis(benzylidene)cyclohexanones for antitumor and cytotoxic activity and as inhibitors of mitochondrial function in yeast: Metabolism studies of (E)-2-benzylidenecyclohexanone. J. Pharm. Sci. 1976, 65, 538–543. [Google Scholar] [CrossRef]
- Dhar, D.N.; Barton, D. The Chemistry of Chalcones and Related Compounds; J. Wiley & Sons: New York, NY, USA, 1981; p. 8. [Google Scholar]
- Gall, E.L.; Texier-Boullet, F.; Hamelin, J. Simple Access to α, β Unsaturated Ketones by Acid-Catalyzed Solvent-Free Reactions. Synth. Commun. 1999, 29, 3651–3657. [Google Scholar] [CrossRef]
- Geissman, T.A.; Clinton, R.O. Flavanones and Related Compounds. I. The Preparation of Polyhydroxychalcones and Flavanones. J. Am. Chem. Soc. 1946, 68, 697–700. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, A.K.; Paul, S.; Kachroo, P.L. Improved Microwave-Induced Synthesis of Chalcones and Related Enones. Indian J. Chem. Sect. B 1995, 34, 61–62. [Google Scholar]
- Lin, T.; Cromwell, N.H.; Kingsbury, C.A. The synthesis and chemistry of a bispyrazolinyl ketone and the structure determination. J. Heterocycl. Chem. 1985, 22, 21–24. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pani, G.; Piermatti, O.; Pizz, F. Condensation reactions in water of active methylene compounds with arylaldehydes. One-pot synthesis of flavonols. Tetrahedron 1994, 50, 11499–11508. [Google Scholar] [CrossRef]
- Li, J.-T.; Chen, G.-F.; Wang, X.-J.; Li, T.-S. Ultrasound Promoted Synthesis of α,α'-bis(Substituted Furfurylidene) Cycloalkanones and Chalcones. Synth. Commun. 1999, 29, 965–971. [Google Scholar] [CrossRef]
- Sinistierra, J.V.; Garcia-Raso, A.; Cabello, J.A.; Marinas, J.M. An Improved Procedure for the Claisen-Schmidt Reaction. Synthesis 1984, 6, 502–504. [Google Scholar]
- Sun, Y.-F.; Wang, Z.-Y.; Zhao, X.; Zheng, Z.-B.; Li, J.-K.; Wu, R.-T.; Cui, Y.-P. The synthesis, spectroscopic characterization and structure of three bis(arylmethylidene)cyclopentanones. Dyes Pigments 2010, 86, 97–105. [Google Scholar] [CrossRef]
- Raston, C.L.G.; Cave, W.V. Green Chemistry Laboratory: Benign Synthesis of 4,6-Diphenyl[2,2']bipyridine via Sequential Solventless Aldol and Michael Addition Reactions. J. Chem. Educ. 2005, 82, 468. [Google Scholar] [CrossRef]
- Raston, C.L.; Scott, J.L. Chemoselective, solvent-free aldol condensation reaction. Green Chem. 2000, 2, 49–52. [Google Scholar] [CrossRef]
- Mogilailah, K.; Swamy, T.K.; Chandra, A.V.; Srivani, N.; Vidya, K. Claisen—Schmidt Condensation under Solvent-Free Conditions. Indian J. Chem. Sec. B 2010, 49, 382–385. [Google Scholar]
- Shan, Z.-X.; Luo, X.-X.; Hu, L.; Hu, X.-Y. New observation on a class of old reactions: Chemoselectivity for the solvent-free reaction of aromatic aldehydes with alkylketones catalyzed by a double-component inorganic base system. Sci. China. Chem. 2010, 53, 1095–1101. [Google Scholar] [CrossRef]
- Nakano, T.; Irifune, S.J.; Umano, S.; Inada, A.; Ishii, Y.; Ogawa, M. Cross-condensation reactions of cycloalkanones with aldehydes and primary alcohols under the influence of zirconocene complexes. J. Org. Chem. 1987, 52, 2239–2244. [Google Scholar] [CrossRef]
- Nakano, T.; Migita, T. A Convenient Synthesis of α,α′-Bis(substitutedbenzylidene)cycloalkanones. Chem. Lett. 1993, 12, 2157–2158. [Google Scholar]
- Zheng, M.; Wang, L.; Shao, J.; Zhong, Q. A Facile Synthesis of α, α'-bis(Substituted Benzylidene)cycloalkanones Catalyzed by bis(p-methoxyphenyl)telluroxide(bmpto) Under Microwave Irradiation. Synth. Commun. 1997, 27, 351–354. [Google Scholar] [CrossRef]
- Iranpoor, N.; Kazemi, F. RuCI3 Catalyses Aldol Condensations of Aldehydes and Ketones. Tetrahedron 1998, 54, 9475–9480. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Y.; Ying, T. A Facile Route to Synthesize α,α′-bis(Substituted-benzylidene) Cycloalkanones Promoted by SmI3. Synth. Commun. 1996, 26, 503–507. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y. SmI3 Catalyzed Condensation of Aliphatic Cycloketones and Aldehydes in Ionic Liquid. Synth. Commun. 2003, 33, 161–165. [Google Scholar] [CrossRef]
- Iranpoor, N.; Zeynizadeh, B.; Aghapour, A. Aldol Condensation of Cycloalkanones with Aromatic Aldehydes Catalysed with TiCl3(SO3CF3). J. Chem. Res, Synop 1999, 9, 554–555. [Google Scholar]
- Dewa, T.; Saiki, T.; Aoyama, Y. Enolization and Aldol Reactions of Ketone with a La3+-Immobilized Organic Solid in Water. A Microporous Enolase Mimic. J. Am. Chem. Soc. 2001, 123, 502–503. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Nagaraju, A.; Sarma, J.A.R.P. Microwave assisted synthesis of α,α′-bis(benzylidene)ketones in dry media. Synth. Commun. 2002, 32, 893–896. [Google Scholar] [CrossRef]
- Salehi, P.; Khodaei, M.M.; Zolfigol, M.A.; Keyvan, A. Solvent-Free Crossed Aldol Condensation of Ketones with Aromatic Aldehydes Mediated by Magnesium Hydrogensulfate. Monatsh. Chem. 2002, 133, 1291–1295. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Niu, H.; Wang, J. An ionic liquid as a recyclable medium for the green preparation of α,α′-bis (substituted benzylidene)cycloalkanones catalyzed by FeCl3·6H2O. Green Chem. 2003, 5, 267–269. [Google Scholar] [CrossRef]
- Huang, D.F.; Wang, J.X.; Hu, Y.L. A New Solvent-free Synthesis of α,α′-Dibenzylidene-cycloalkanones from Acetals with Cycloalkanones under Microwave Irradiation. Chin. Chem. Lett. 2003, 14, 333–334. [Google Scholar]
- Deng, G.; Ren, T. Indium Trichloride Catalyzes Aldol-Condensations of Aldehydes and Ketones. Synth. Commun. 2003, 33, 2995–3001. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S.K.K.; Reddy, K.B.; Yadav, J.S. Iodotrimethylsilane-Mediated Cross-Aldol Condensation: A Facile Synthesis of α,α′-Bis(substituted benzylidene)cycloalkanones. Synthesis 2004, 263–266. [Google Scholar]
- Zhu, Y.; Pan, Y. A New Lewis Acid System Palladium/TMSCl for Catalytic Aldol Condensation of Aldehydes with Ketones. Chem. Lett. 2004, 33, 668–669. [Google Scholar] [CrossRef]
- Hu, Z.G.; Liu, J.; Zeng, P.L.; Dong, Z.B. Synthesis of α,α′-Bis(substituted benzylidene)ketones Catalyzed by a SOCl2/EtOH Reagent. J. Chem. Res. Synop. 2004, 1, 55–56. [Google Scholar]
- Wang, L.; Sheng, J.; Tian, H.; Han, J.; Fan, Z.; Qian, C. A Convenient Synthesis of α,α′-Bis(substituted benzylidene)cycloalkanones Catalyzed by Yb(OTf)3 Under Solvent-Free Conditions. Synthesis 2004, 18, 3060–3064. [Google Scholar]
- Cao, Y.-Q.; Zhi, D.; Zhang, R.; Chen, B.-H. Aldol Condensations Catalyzed by PEG400 and Anhydrous K2CO3 without Solvent. Synth. Commun. 2005, 35, 1045–1049. [Google Scholar] [CrossRef]
- Das, B.; Thirupathi, P.; Mahender, I.; Reddy, K.R. Convenient and facile cross-Aldol condensation catalyzed by molecular iodine: An efficient synthesis of α,α′-bis(substituted-benzylidene) cycloalkanones. J. Mol. Catal. A Chem. 2006, 247, 182–185. [Google Scholar] [CrossRef]
- Li, J.; Su, W.; Li, N. Copper Triflate–Catalyzed Cross‐Aldol Condensation: A Facile Synthesis of α,α′‐Bis(Substituted Benzylidene) Cycloalkanones. Synth. Commun. 2005, 35, 3037–3043. [Google Scholar] [CrossRef]
- Hazarkhani, H.; Kumar, P.; Kondiram, K.S.; Shafi Gadwal, I.M. Highly Selective Claisen-Schmidt Condensation Catalyzed by Silica Chloride Under Solvent-Free Reaction Conditions. Synth. Commun. 2010, 40, 2887–2896. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Zare, A.; Balooty, L.; Mehregan, M.; Shekouhy, M. Solvent-Free, Cross-Aldol Condensation Reaction Using Silica-Supported, Phosphorus-Containing Reagents Leading to α,α′-Bis(arylidene)cycloalkanones. Synth. Commun. 2010, 40, 3488–3495. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, X.-M.; Pang, L.-L.; Ma, R.; Yue, C.-H.; Yuan, R.; Lin, W.; Yin, W.; Bo, R.-C.; Wu, H. Synthesis and Fluorescence Properties of α,α′-Bis(substituted-benzylidene)cycloalkanones Catalyzed by 1-Methyl-3(2-(sulfooxy)ethyl)-1H-imidazol-3-ium Chloride. Synth. Commun. 2010, 40, 2320–2328. [Google Scholar] [CrossRef]
- Arnold, A.; Markert, M.; Mahrwald, R. Amine-Catalyzed Aldol Condensation in the Presence of Lithium Perchlorate. Synthesis 2006, 7, 1099–1102. [Google Scholar]
- Riadi, Y.; Mamouni, R.; Azzalou, R.; Boulahjar, R.; Abrouki, Y.; Haddad, M.El.; Routier, S. Animal bone meal as an efficient catalyst for crossed-aldol condensation. Tet. Lett. 2010, 51, 6715–6717. [Google Scholar] [CrossRef]
- Yang, S.-D.; Wu, L.-Y.; Yan, Z.-Y.; Pan, Z.-L.; Liang, Y.-M. A novel ionic liquid supported organocatalyst of pyrrolidine amide: Synthesis and catalyzed Claisen-Schmidt. J. Mol. Catal. A Chem. 2007, 268, 107–111. [Google Scholar] [CrossRef]
- Kang, K.-Q.; Song, G.-H.; Wang, J.-Y.; Wei, B.-G. Synthesis of α,α′-Bis(substituted benzylidene)cycloalkanones Catalyzed by Amino-Functionalized Ionic Liquid. J. Chin. Chem. Soc. 2008, 55, 1125–1128. [Google Scholar]
- Solhy, A.; Amer, W.; Karkouri, M.; Tahir, R.; El Bouari, A.; Fihri, A.; Bousmina, M.; Zahouily, M. Bi-functional modified-phosphate catalyzed the synthesis of α-α′-(EE)-bis(benzylidene)-cycloalkanones: Microwave versus conventional-heating. Mol. Catal. A Chem. 2011, 336, 8–15. [Google Scholar] [CrossRef]
- Shrikhande, J.J.; Gawande, M.B.; Jayaram, R.V. Cross-aldol and Knoevenagel condensation reactions in aqueous micellar media. Catal. Commun. 2008, 9, 1010–1016. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, J.; Yadav, A.; Chaturvedi, V.; Bhatnagar, S.; Gaikwad, A.N.; Sinha, S.K.; Kumar, A.; Shukla, P.K.; Tripathi, R.P. A facile synthesis of α,α′-(EE)-bis(benzylidene)-cycloalkanones and their antitubercular evaluations. Eur. J. Med. Chem. 2009, 44, 1705–1709. [Google Scholar] [CrossRef]
- Bigdeli, M.A.; Mahdavinia, G.H.; Jafari, S.; Hazarkhani, H. Wet 2,4,6-trichloro[1,3,5]triazine (TCT) an efficient catalyst for synthesis of α,α′-bis(substituted-benzylidene) cycloalkanones under solvent-free conditions. Catal. Commun. 2007, 8, 2229–2231. [Google Scholar] [CrossRef]
- An, L.T.; Zou, J.P.; Zhang, L.L. Polymer-supported sulphonic acid catalyzed cross-aldol condensation: An expeditious synthesis of α,α′-bis(substituted benzylidene) cycloalkanones. Catal. Commun. 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Bhagat, S.; Sharma, R.; Chakraborti, A.K. Dual-activation protocol for tandem cross-aldol condensation: An easy and highly efficient synthesis of α,α′-bis(aryl/alkylmethylidene)ketones. J. Mol. Catal. A Chem. 2006, 260, 235–240. [Google Scholar] [CrossRef]
- Yi, W.B.; Cai, C. Aldol condensations of aldehydes and ketones catalyzed by rare earth(III) perfluorooctane sulfonates in fluorous solvents. J. Fluorine Chem. 2005, 126, 1553–1558. [Google Scholar] [CrossRef]
- Schriner, L.; Kurosawa, T. Chalcones. ii. decomposition by alkali. J. Am. Chem. Soc. 1930, 52, 2538–2540. [Google Scholar] [CrossRef]
- Dhar, D.N.; Lal, J.B. Chalcones. Condensation of Aromatic Aldehydes with Resacetophenone II. J. Org. Chem. 1958, 23, 1159–1161. [Google Scholar] [CrossRef]
- Hathaway, B.A. An aldol condensation experiment using a number of aldehydes and ketones. J. Chem. Educ. 1987, 64, 367. [Google Scholar] [CrossRef]
- Irie, K.; Watanabe, K. Aldol Condensations with Metal(II) Complex Catalysts. Bull. Chem. Soc. Jpn. 1980, 53, 1366–1371. [Google Scholar] [CrossRef]
- Rahman, M.A.F.M.; Jeong, B.S.; Kim, D.H.; Park, J.K.; Lee, E.S.; Jahng, Y. A facile synthesis of α,α′-bis(substituted-benzylidene)-cycloalkanones and substituted-benzylidene heteroaromatics: utility of NaOAc as a catalyst for aldol-type reaction. Tetrahedron 2007, 63, 2426–2431. [Google Scholar] [CrossRef]
- Kim, D.H.; Rahman, M.A.F.M.; Jeong, B.S.; Lee, E.S.; Jahng, Y. Acetate-Promoted Aldol-Type Reaction: Scope and Reactivity of Acetates and Aldehydes. Bull. Kor. Chem. Soc. 2009, 30, 797–802. [Google Scholar] [CrossRef]
- Garland, C.E.; Reid, E.E. Some new derivatives of cyclohexanone. J. Am. Chem. Soc. 1925, 47, 2333–2340. [Google Scholar] [CrossRef]
- English, J., Jr.; Lamberti, V. Optically Active 1-Cyclohexenyl- and 1-Cyclopentenylmethylcarbinols. J. Am. Chem. Soc. 1952, 74, 1909–1912. [Google Scholar] [CrossRef]
- Murakaiyama, T.; Banno, K.; Narasaka, K. New cross-aldol reactions. Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride. J. Am. Chem. Soc. 1974, 96, 7503–7509. [Google Scholar] [CrossRef]
- Tully, W.; Main, L.; Nicholson, B.K. β-Cyclomanganated 1,5-diphenylpenta-1,4-dien-3-ones and their reactions with alkynes: Routes to η5-pyranyl and η5-oxocycloheptadienylMn(CO)3 complexes. J. Organomet. Chem. 2001, 633, 162–172. [Google Scholar] [CrossRef]
- Vogel, A.I. Practical Organic Chemistry, 3rd ed; Longman: London, UK, 1956; p. 718. [Google Scholar]
- Kossanyi, J.; Furth, B.; Morizur, J.P. Influence de l'insaturation en série bicyclique pontée: Transposition de l'acétate de trans-benzylidène-3 isobornyle. Tetrahedron 1970, 26, 395–409. [Google Scholar] [CrossRef]
- Groselj, U.; Bevk, D.; Jakse, R.; Meden, A.; Stanovnik, B.; Svete, J. Stereoselective additions to the exocyclic C=C bond of some α-alkylidene-(+)-camphor derivatives. Tetrahedron: Asymmetry 2006, 17, 1217–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rahman, A.F.M.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571-583. https://doi.org/10.3390/molecules17010571
Rahman AFMM, Ali R, Jahng Y, Kadi AA. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules. 2012; 17(1):571-583. https://doi.org/10.3390/molecules17010571
Chicago/Turabian StyleRahman, A. F. M. Motiur, Roushown Ali, Yurngdong Jahng, and Adnan A. Kadi. 2012. "A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones" Molecules 17, no. 1: 571-583. https://doi.org/10.3390/molecules17010571
APA StyleRahman, A. F. M. M., Ali, R., Jahng, Y., & Kadi, A. A. (2012). A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules, 17(1), 571-583. https://doi.org/10.3390/molecules17010571