Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid
Abstract
:1. Introduction

2. Results and Discussion
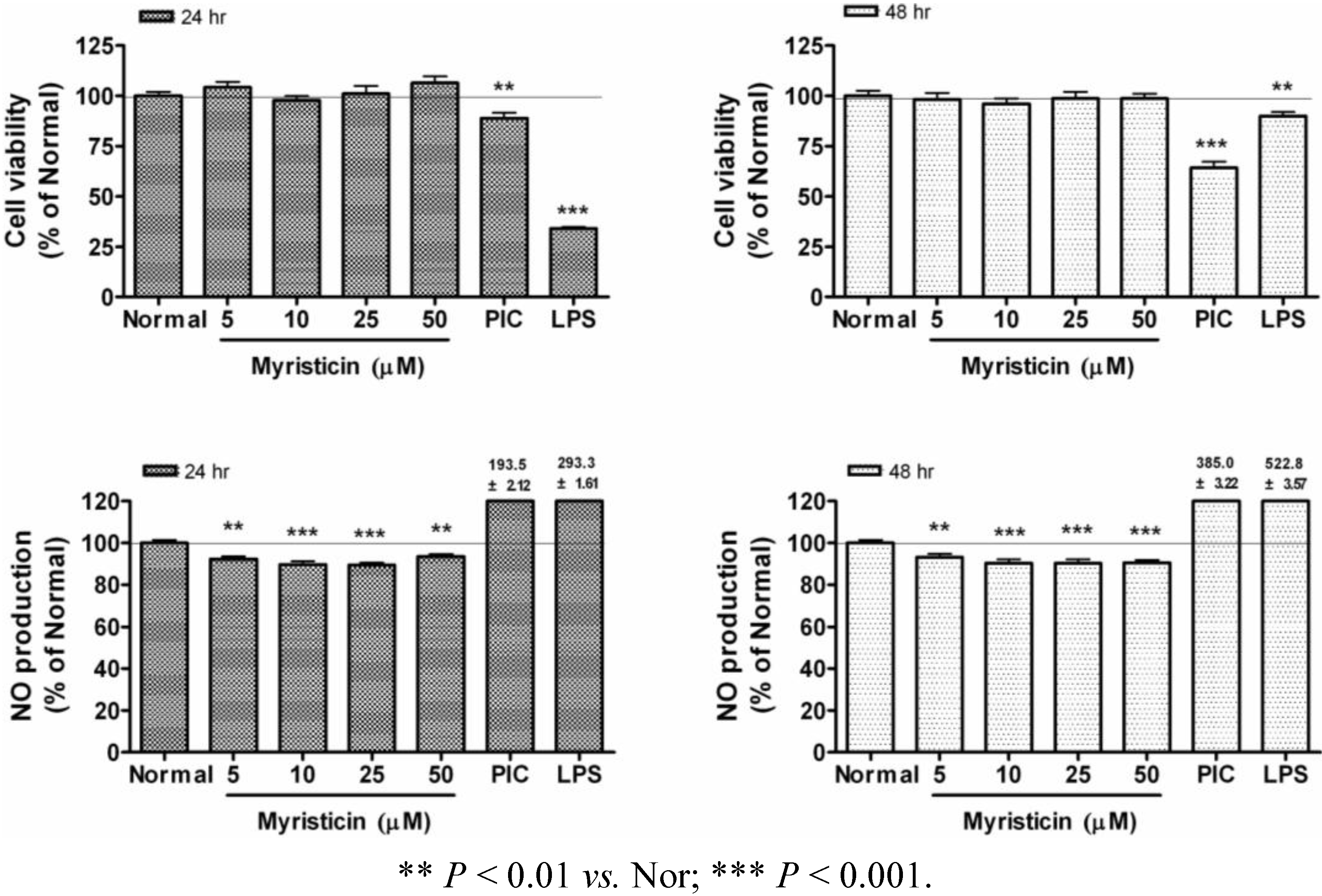
2.1. Effects of Myristicin on Cell Viability
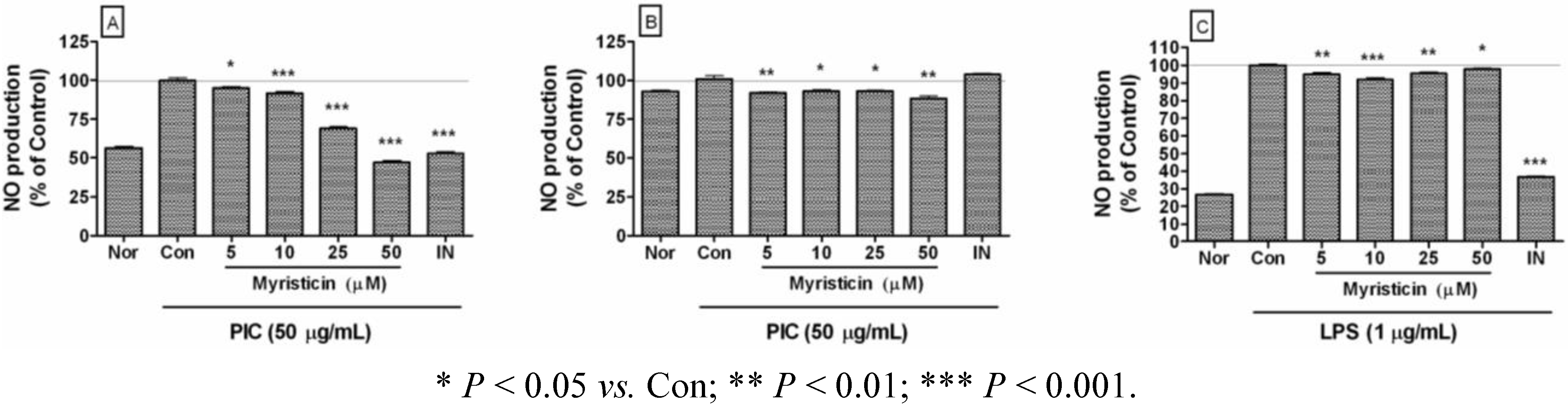
2.2. Effects of Myristicin on NO Production
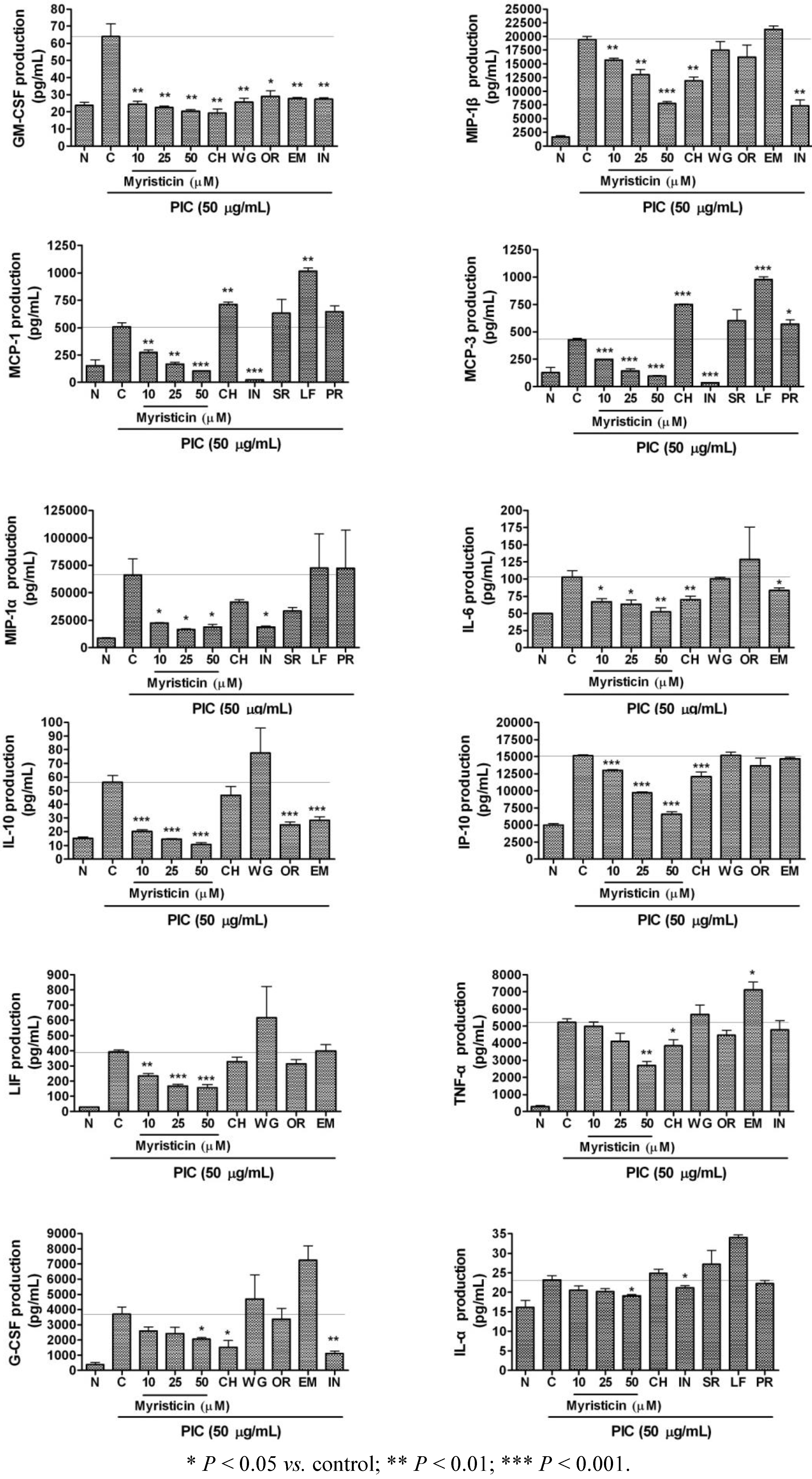
2.3. Effects of Myristicin on Cytokine Production
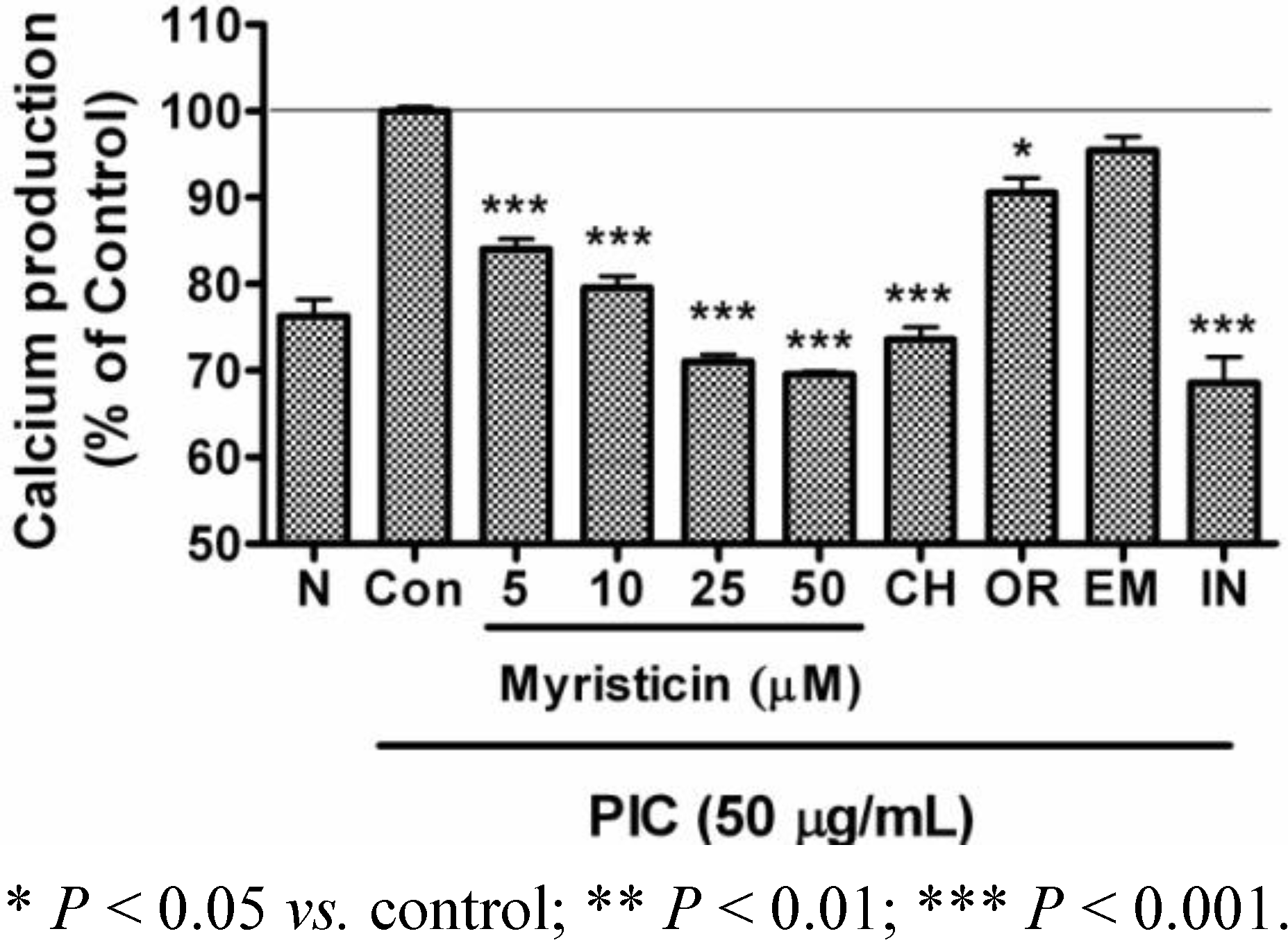
2.4. Effects of Myristicin on Intracellular Calcium Production
3. Experimental
3.1. Reagents
3.2. Cell Culture and Viability
3.3. Quantification of NO Production
3.4. Multiplex Bead-Based Cytokine Assay
3.5. Intracellular Calcium Assay
3.6. Statistical Analysis
4. Conclusions
Conflict of Interest
Acknowledgements
References and Notes
- Lee, B.K.; Kim, J.H.; Jung, J.W.; Choi, J.W.; Han, E.S.; Lee, S.H.; Ko, K.H.; Ryu, J.H. Myristicin-induced neurotoxicity in human neuroblastoma SK-N-SH cells. Toxicol. Lett. 2005, 157, 49–56. [Google Scholar] [CrossRef]
- Martins, C.; Doran, C.; Laires, A.; Rueff, J.; Rodrigues, A.S. Genotoxic and apoptotic activities of the food flavourings myristicin and eugenol in AA8 and XRCC1 deficient EM9 cells. Food Chem. Toxicol. 2011, 49, 385–392. [Google Scholar] [CrossRef]
- Hallström, H.; Thuvander, A. Toxicological evaluation of myristicin. Nat. Toxins 1997, 5, 186–192. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Slamenova, D.; Horvathova, E.; Wsolova, L.; Sramkova, M.; Navarova, J. Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat. Res. 2009, 677, 46–52. [Google Scholar] [CrossRef]
- Narasimhan, B.; Dhake, A.S. Antibacterial principles from Myristica fragrans seeds. J. Med. Food 2006, 9, 395–399. [Google Scholar] [CrossRef]
- Ferret, P.J.; Soum, E.; Negre, O.; Fradelizi, D. Auto-protective redox buffering systems in stimulated macrophages. BMC Immunol. 2002, 3, 3. [Google Scholar] [CrossRef]
- O'Shea, J.J.; Ma, A.; Lipsky, P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002, 2, 37–45. [Google Scholar] [CrossRef]
- Ware, C.F. Network communications: Lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 2005, 23, 787–819. [Google Scholar] [CrossRef]
- Ulevitch, R.J.; Tobias, P.S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 1995, 13, 437–457. [Google Scholar] [CrossRef]
- Blair, L.A.; Maggi, L.B., Jr.; Scarim, A.L.; Corbett, J.A. Role of interferon regulatory factor-1 in double-stranded RNA-induced iNOS expression by mouse islets. J. Biol.Chem. 2002, 277, 359–365. [Google Scholar]
- Pereira, R.M.; Teixeira, K.L.; Barreto-de-Souza, V.; Calegari-Silva, T.C.; De-Melo, L.D.; Soares, D.C.; Bou-Habib, D.C.; Silva, A.M.; Saraiva, E.M.; Lopes, U.G. Novel role for the double-stranded RNA-activated protein kinase PKR: Modulation of macrophage infection by the protozoan parasite Leishmania. FASEB J. 2010, 24, 617–626. [Google Scholar]
- Jiang, W.; Bell, C.W.; Pisetsky, D.S. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J. Immunol. 2007, 178, 6495–6503. [Google Scholar]
- Narasimhan, B.; Dhake, A.S. Antibacterial principles from Myristica fragrans seeds. J. Med. Food 2006, 9, 395–399. [Google Scholar] [CrossRef]
- Ahmad, H.; Tijerina, M.T.; Tobola, A.S. Preferential overexpression of a class MU glutathione S-transferase subunit in mouse liver by myristicin. Biochem. Biophys. Res. Commun. 1997, 236, 825–828. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Kenney, P.M.; Zhang, J.; Lam, L.K. Inhibition of benzo[a]pyrene-induced tumorigenesis by myristicin, a volatile aroma constituent of parsley leaf oil. Carcinogenesis 1992, 13, 1921–1923. [Google Scholar] [CrossRef]
- Morita, T.; Jinno, K.; Kawagishi, H.; Arimoto, Y.; Suganuma, H.; Inakuma, T.; Sugiyama, K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/dgalactosamine-induced liver injury. J. Agric. Food Chem. 2003, 51, 1560–1565. [Google Scholar]
- Lee, B.K.; Kim, J.H.; Jung, J.W.; Choi, J.W.; Han, E.S.; Lee, S.H.; Ko, K.H.; Ryu, J.H. Myristicin-induced neurotoxicity in human neuroblastoma SK-N-SH cells. Toxicol. Lett. 2005, 157, 49–56. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J. Ethnopharmacol. 2007, 114, 78–85. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van, Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and proresolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Jiang, W.; Bell, C.W.; Pisetsky, D.S. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J. Immunol. 2007, 178, 6495–6503. [Google Scholar]
- Ito, H. IL-6 and Crohn's disease. Curr. Drug Targets Inflamm. Allergy 2003, 2, 125–130. [Google Scholar] [CrossRef]
- Guimbaud, R.; Abitbol, V.; Bertrand, V.; Quartier, G.; Chauvelot-Moachon, L.; Giroud, J.; Couturier, D.; Chaussade, D.C. Leukemia inhibitory factor involvement in human ulcerative colitis and its potential role in malignant course. Eur. Cytokine. Netw. 1998, 9, 607–612. [Google Scholar]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine. Growth. Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Adcock, I.M.; Caramori, G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 2001, 79, 376–384. [Google Scholar] [CrossRef]
- White, K.E.; Ding, Q.; Moore, B.B.; Peters-Golden, M.; Ware, L.B.; Matthay, M.A.; Olman, M.A. Prostaglandin E2 mediates IL-1beta-related fibroblast mitogenic effects in acute lung injury through differential utilization of prostanoid receptors. J. Immunol. 2008, 180, 637–646. [Google Scholar]
- Srivastava, M.; Jung, S.; Wilhelm, J.; Fink, L.; Bühling, F.; Welte, T.; Bohle, R.M.; Seeger, W.; Lohmeyer, J.; Maus, U.A. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J. Immunol. 2005, 175, 1884–1893. [Google Scholar]
- Zhang, Y.; Xu, C.B.; Cardell, L.O. Long-term exposure to IL-1beta enhances Toll-IL-1 receptor-mediated inflammatory signaling in murine airway hyperresponsiveness. Eur. Cytokine. Netw. 2009, 20, 148–156. [Google Scholar]
- Barnes, P.J.; Chung, K.F.; Page, C.P. Inflammatory mediators of asthma: An update. Pharmacol. Rev. 1998, 50, 515–596. [Google Scholar]
- Cuschieri, J.; Maier, R.V. Oxidative stress, lipid rafts, and macrophage reprogramming. Antioxid. Redox. Signal. 2007, 9, 1485–1497. [Google Scholar] [CrossRef]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 activation causes translocation of Bax to the endoplasmic reticulum during the resolution of airway mucous cell hyperplasia by IFN-gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar]
- Willems, T.; Lefebvre, D.J.; Neyts, J.; De Clercq, K. Diagnostic performance and application of two commercial cell viability assays in foot-and-mouth disease research. J. Virol. Methods 2011, 173, 108–114. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Yuk, S.S.; Lim, E.M.; Lee, J.Y.; Lee, Y.J.; Kim, Y.S.; Lee, T.H.; Park, S.K.; Bae, H.; Kim, H.M.; Ko, S.G.; Oh, M.S.; Park, W. Antiinflammatory effects of Epimedium brevicornum water extract on lipopolysaccharide-activated RAW264.7 macrophages. Phytother. R. 2010, 24, 1781–1787. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid. Molecules 2011, 16, 7132-7142. https://doi.org/10.3390/molecules16087132
Lee JY, Park W. Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid. Molecules. 2011; 16(8):7132-7142. https://doi.org/10.3390/molecules16087132
Chicago/Turabian StyleLee, Ji Young, and Wansu Park. 2011. "Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid" Molecules 16, no. 8: 7132-7142. https://doi.org/10.3390/molecules16087132
APA StyleLee, J. Y., & Park, W. (2011). Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid. Molecules, 16(8), 7132-7142. https://doi.org/10.3390/molecules16087132