Friedel-Craft Acylation of ar-Himachalene: Synthesis of Acyl-ar-Himachalene and a New Acyl-Hydroperoxide
Abstract
:1. Introduction

2. Results and Discussion
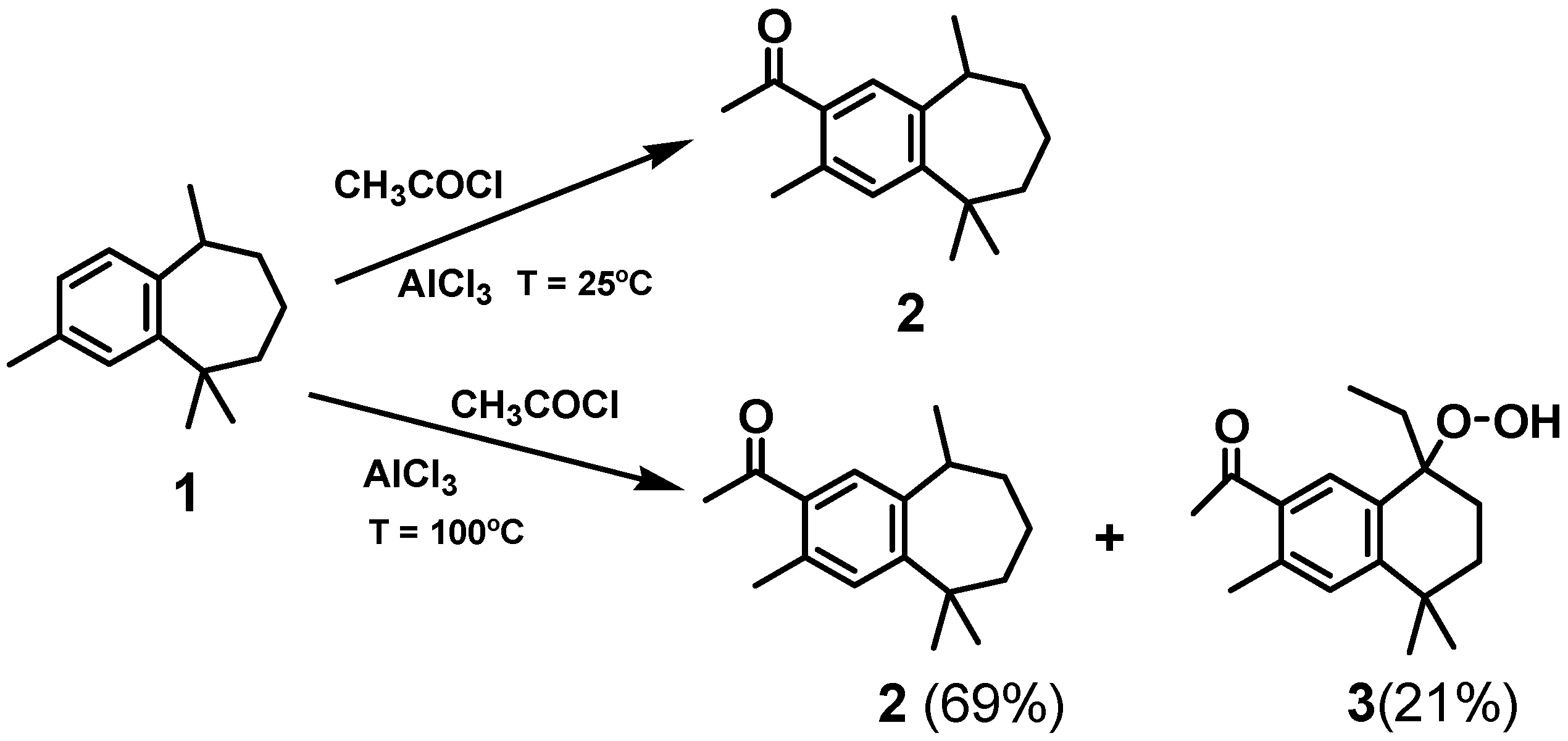
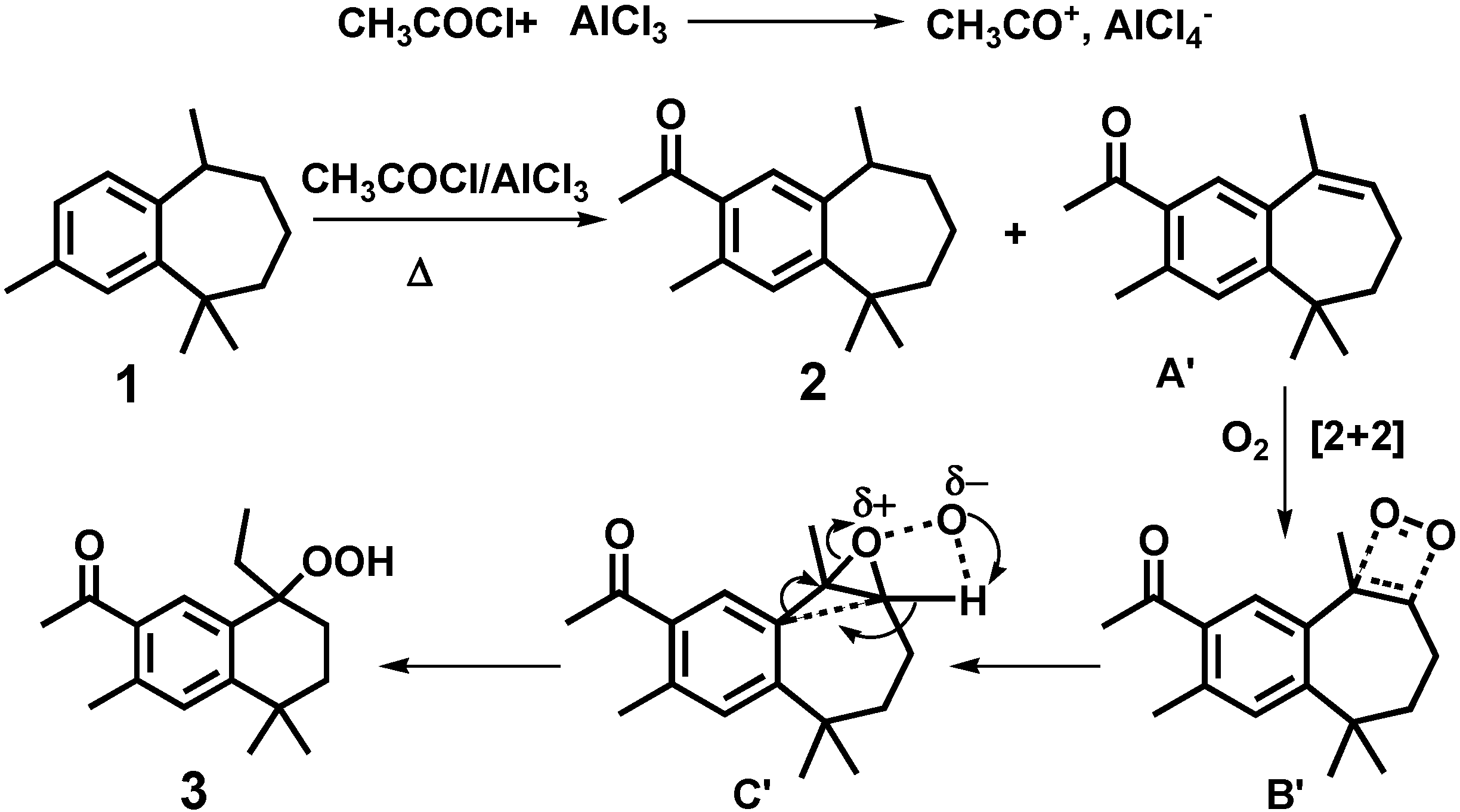

| O(1)-O(2) | 1.462(3) | O(1)-C(8)-C(9) | 109.8(2) |
| O(1)-C(8) | 1.452(3) | O(1)-C(8)-C(16) | 102.4(2) |
| O(3)-C(11) | 1.232(3) | O(1)-C(8)-C(7) | 110.2(2) |
| O(2)-O(1)-C(8) | 108.73(19) | O(3)-C(11)-C(1) | 120.8(3) |
| D--H..A | d(D--H) | d(H..A) | d(D....A) | angle (D--H..A) | Symmetry label |
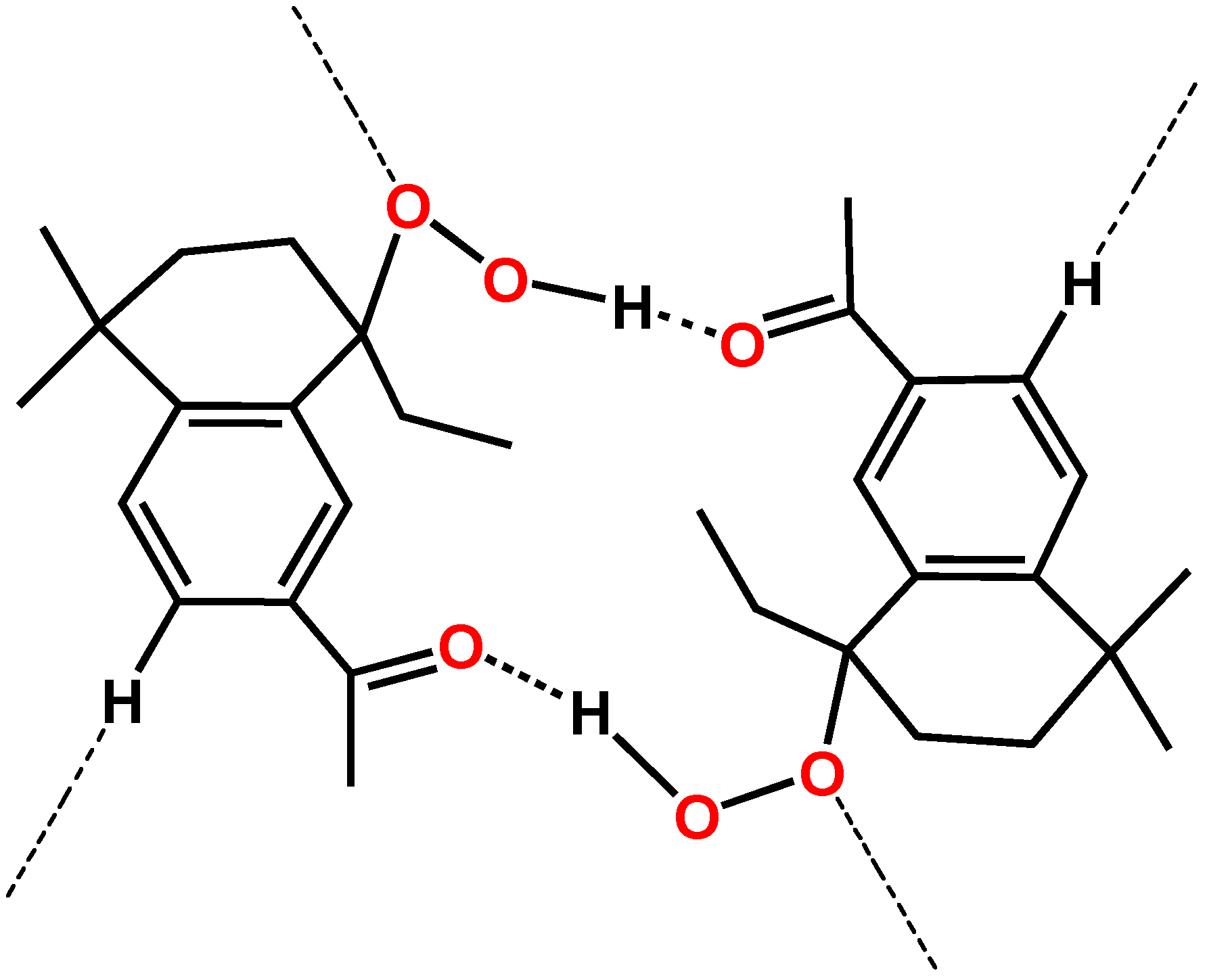
| O(2)--H(2A)..O(3) | 0.8400 | 1.9400 | 2.765(3) | 165.00 | 3_556 |
| C(3)--H(3)..O(1) | 0.9500 | 2.4800 | 3.321(4) | 147.00 | 4_555 |
| C(7)--H(7B)..O(2) | 0.9900 | 2.3700 | 2.806(4) | 106.00 | intramolecular |

3. Experimental
3.1. General
3.2. General Acylation Procedure:
3.3. Acyl-Ar-Himachalene 2:
3.4. Acyl-Hydroperoxyde 3:
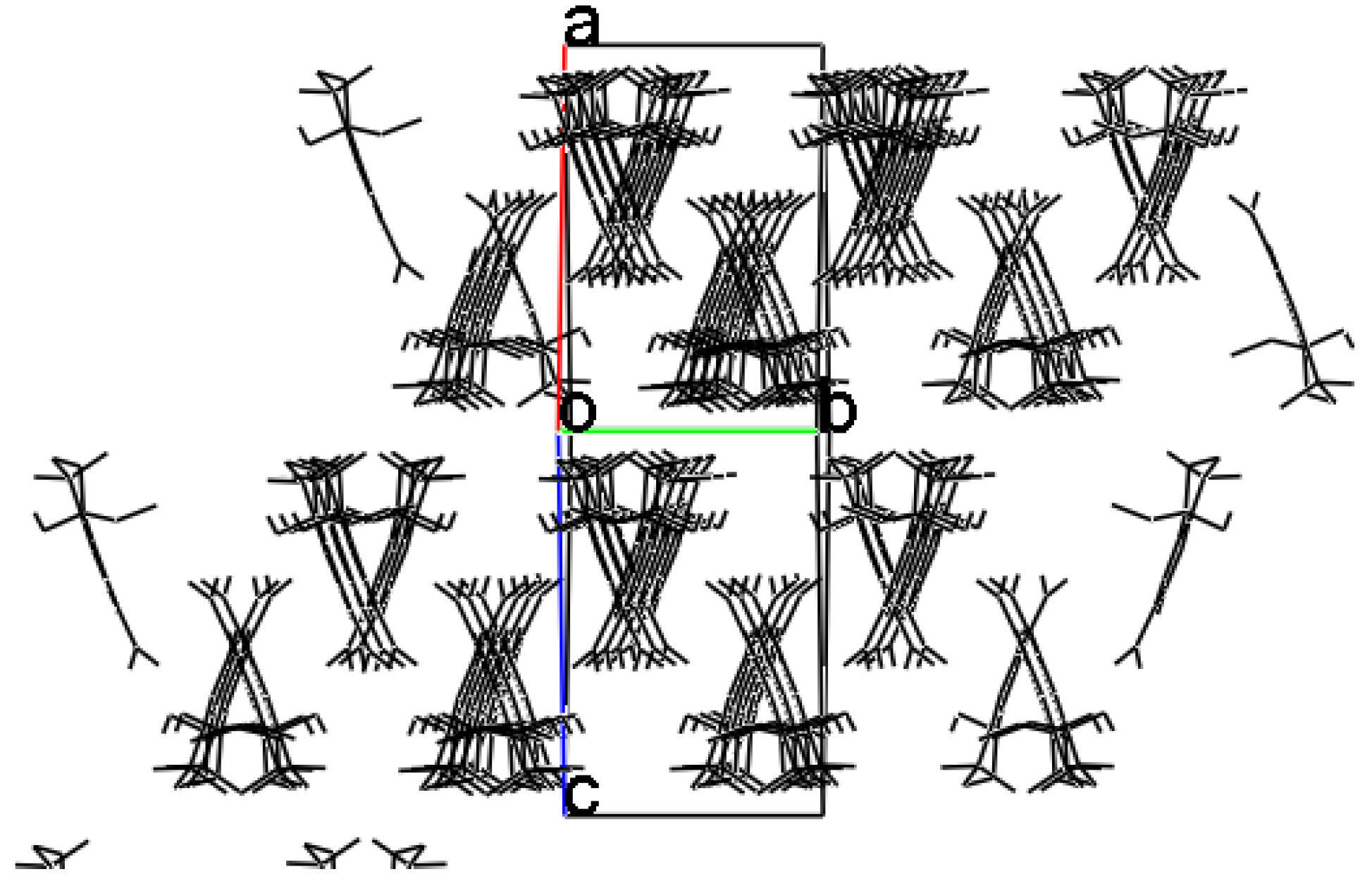
3.5. X-ray Structure Determination
| Formula | C17 H24 O3 |
| Formula Weight | 276.36 |
| Crystal System | Monoclinic |
| Space group | P21/n (No. 14) |
| a, b, c (Å) | 14.459(9), 8.166(5), 14.459(9) |
| α, β, γ (°) | 90, 115.50(2), 90 |
| V (Å3) | 1540.9(17) |
| Z | 4 |
| d(calc) (g/cm3) | 1.191 |
| µ (MoKα) (mm−1) | 0.080 |
| F(000) | 600 |
| Crystal Size (mm) | 0.27 × 0.54 × 0.56 |
| Temperature (K) | 100 |
| Radiation, λ (Å) | MoKα, 0.71073 |
| θ Min-Max (°) | 1.7, 25.0 |
| Dataset (h, k, l ranges) | −17:16; −9:7; −17:17 |
| Tot., Uniq. Data, R(int) | 7525, 2657, 0.188 |
| Observed data [I > 2.0 σ(I)] | 2236 |
| Nref, Npar | 2657, 187 |
| R, wR2, S * | 0.0703, 0.1937, 1.06 |
| Max. and Av. Shift/Error | 0.000, 0.000 |
| Min. and Max. Resd. Dens. (e/A3) | −0.31, 0.31 |
4. Conclusions
Acknowledgements
References
- Adams, R.P. Cedar Wood oil–analysis and properties. In Modern Methods of Plant Analysis, New Series, Vol. 12, Essential Oils and Waxes; Linskens, H.F., Jackson, J.F., Eds.; Springer-Verlag: New York, NY, USA, 1991; pp. 159–173. [Google Scholar]
- Digrak, M.; Ilcim, A.; Hakki, A.M. Antimicrobial activities of several parts of Pinus brutia, Juniperus oxycedrus, Abies Cilicia, Cedrus libani and Pinus nigra. Phytother. Res. 1999, 13, 584–587. [Google Scholar] [CrossRef]
- Singh, D.; Rao, S.M.; Tripathi, A.K. Cedarwood oil [Cedrus deodara] as a potential insecticidal agent against mosquitoes. Naturwissenschaften 1984, 71, 265–266. [Google Scholar]
- Walker, G.T. Cedarwood oil. Perfum. Essent. Oil Rec. 1968, 59, 347–350. [Google Scholar]
- Lawrence, B.M. Cedarwood oil. Perfum. Flavor. 1980, 5, 63. [Google Scholar]
- Mori, K. Recent results in the synthesis of ecologically important bioregulators. Pure Appl. Chem. 2001, 73, 601–606. [Google Scholar] [CrossRef]
- Chalchat, J.-C.; Garry, R.-P.; Michet, A. Essential oil in sawdust of Cedrus atlantica from Morocco. JOER 1994, 6, 323–325. [Google Scholar]
- Balchin, L. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragrance J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cossé, A.A.; Zilkowski, B.W.; Weisleder, D.; Momany, F.A. Male-specific sesquiterpenes from Phyllotreta and Aphthona flea beetles. J. Chem. Ecol. 2001, 27, 2397–2423. [Google Scholar] [CrossRef]
- Mori, K. Synthesis of (R)-ar-turmerone and its conversion to (R)-ar-himachalene, a pheromone component of the flea beetle: (R)-ar-himachalene is dextrorotatory in hexane, while levorotatory in chloroform. Tetrahedron: Asymmetry 2005, 16, 685–692. [Google Scholar] [CrossRef]
- El Firdoussi, L.; Benharref, A.; Allaoud, S.; Karim, A.; Castanet, Y.; Mortreux, A.; Petit, F. Palladium(II)-catalyzed acetoxylation and methoxylation of olefinic terpenes. J. Mol. Catal. 1992, 72, L1–L5. [Google Scholar] [CrossRef]
- El Firdoussi, L.; Baqqa, A.; Allaoud, S.; Ait Allal, B.; Karim, A.; Castanet, Y.; Mortreux, A. Selective palladium-catalyzed functionalization of limonene: synthetic and mechanistic aspects. J. Mol. Catal. A: Chem. 1998, 135, 11–22. [Google Scholar] [CrossRef]
- Ait Allal, B.; El Firdoussi, L.; Allaoud, S.; Karim, A.; Castanet, Y.; Mortreux, A. J. Mol. Catal. A: Chem. 2000, 200, 177–184.
- Ziyat, H.; El Houssame, S.; Ait Ali, M.Y.; Ait Itto, M.; Karim, A.; Wartchow, R.; Butenschon, H. Synthesis, structure, and absolute configuration of a new cyclopropanic compound derived from the sesquiterpene β-himachalene. Z. Naturforsch. 2004, 59b, 1177–1179. [Google Scholar]
- Ziyat, H.; Ait Ali, M.; Karim, A.; Meliet, C.; Castanet, Y.; Mortreux, A. Acta Chim. Slov. 2004, 51, 223–230.
- Ziyat, H.; Ait Itto, M.Y.; Ait Ali, M.; Riahi, A.; Karim, A.; Daran, J.-C. Arkivoc 2006, xii, 152–160.
- Feddouli, A.; Ait Itto, M.Y.; Ait Ali, M.; Hasnaoui, A.; Riahi, A. Efficient Approach for the synthesis of novel functionalized isoxazolines from limonene. Synth. Commun. 2006, 36, 3617–3624. [Google Scholar] [CrossRef]
- El Firdoussi, L.; Ait Ali, M.; Karim, A.; Spannenberg, A. Zeitschrift Kristallographie 2007, NCS 222, 195–196.
- Mévellec, V.; Mattioda, C.; Schulz, J.; Rolland, J.P.; Roucoux, A. Enantioselective hydrogenation of ethyl pyruvate in biphasic liquid–liquid media by reusable surfactant-stabilized aqueous suspensions of platinum nanoparticles. J. Catal. 2004, 225, 1–6. [Google Scholar] [CrossRef]
- Gao, W.T.; Zheng, Z. Synthesis and characterization of chiral nitrobenzaldehyde-schiff base ligands. Molecules 2003, 8, 788–792. [Google Scholar] [CrossRef]
- Fehr, C.; Chaptal-Gradoz, N.C. Syntheses of the enantiomers of vulcanolide. Helv. Chim. Acta 2002, 85, 533–543. [Google Scholar] [CrossRef]
- Fehr, C.; Chaptal-Gradoz, N.; Galindo, J. Synthesis of (−)-Vulcanolide by enantioselective protonation. Chem. Eur. J. 2002, 8, 853–858. [Google Scholar] [CrossRef]
- Abouhamza, B.; Allaoud, S.; Karim, A. 2,4-Dimethyl-3-phenyl-[2'-methyl-3'-chloro]-7-chloro-8-methyl-1,3-diaza-2,4-diboranaphtalene Tricarbonylchromium Complexes. Molecules 2001, 6, M211. [Google Scholar]
- Alberti, M.N.; Orfanopoulos, M. Stereoelectronic and solvent effects on the allylic oxyfunctionalization of alkenes with singlet oxygen. Tetrahedron 2006, 62, 10660–10675. [Google Scholar] [CrossRef]
- El Haib, A.; Benharref, A.; Parrès-Maynadié, S.; Manoury, E.; Daran, J.-C.; Urrutigoïty, M.; Gouygou, M. Molecular rearrangement of epoxide derived from sesquiterpenes by Lewis acid catalysis. Tetrahedron: Asymmetry 2010, 21, 1272–1277. [Google Scholar] [CrossRef]
- El Haib, A.; Benharref, A.; Parrès-Maynadié, S.; Manoury, E.; Urrutigoïty, M.; Gouygou, M. Lewis acid- and Bronsted acid-catalyzed stereoselective rearrangement of epoxides derived from himachalenes: Access to new chiral polycyclic structures. Tetrahedron: Asymmetry 2011, 22, 101–108. [Google Scholar] [CrossRef]
- Lee, K.-H.; Nozaki, H.; McPhail, A.T. Structure and stereochemistry of maytensifolin-a, a novel hydroperoxy-nortriterpene from maytenus diversifolia. Tetrahedron Lett. 1984, 25, 707–710. [Google Scholar] [CrossRef]
- Perales, A.; Piozzi, F. The X-ray revised stereochemistry of the hydroperoxide of atractyligenin. Z. Kristallogr. 1989, 188, 255–259. [Google Scholar] [CrossRef]
- Bernat, V.; André-Barrès, C.; Baltas, M.; Saffon, N.; Vial, H. Synthesis of antimalarial G-factors endoperoxides: Relevant evidence of the formation of a biradical during the autoxidation step. Tetrahedron 2008, 64, 9216–9224. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Lex, J.; Saygin, K.M.; Steinwascher, J. Azidohydroperoxidation of pinenes: stereoselectivity pattern and the first X-ray structure of a 2-azidohydroperoxide. Chem. Commun. 2000, 2205–2206. [Google Scholar] [CrossRef]
- Khrustalev; Antipin, N.Yu.; Kosnikov, A.Yu.; Antonovsky, V.L. Krystallografiya. Crystallogr. Rep. 2004, 49, 860. [Google Scholar]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. SHELXTL, Version 6.10, Crystal Structure Analysis Package; Bruker AXS: Madison, WI, USA, 2000. [Google Scholar]
- Farrugia, L.J. ORTEP-3 for Windows, Version 1.076. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hossini, I.; Harrad, M.A.; Ait Ali, M.; El Firdoussi, L.; Karim, A.; Valerga, P.; Puerta, M.C. Friedel-Craft Acylation of ar-Himachalene: Synthesis of Acyl-ar-Himachalene and a New Acyl-Hydroperoxide. Molecules 2011, 16, 5886-5895. https://doi.org/10.3390/molecules16075886
Hossini I, Harrad MA, Ait Ali M, El Firdoussi L, Karim A, Valerga P, Puerta MC. Friedel-Craft Acylation of ar-Himachalene: Synthesis of Acyl-ar-Himachalene and a New Acyl-Hydroperoxide. Molecules. 2011; 16(7):5886-5895. https://doi.org/10.3390/molecules16075886
Chicago/Turabian StyleHossini, Issam, Mohamed Anoir Harrad, Mustapha Ait Ali, Larbi El Firdoussi, Abdallah Karim, Pedro Valerga, and M. Carmen Puerta. 2011. "Friedel-Craft Acylation of ar-Himachalene: Synthesis of Acyl-ar-Himachalene and a New Acyl-Hydroperoxide" Molecules 16, no. 7: 5886-5895. https://doi.org/10.3390/molecules16075886




