Absorbable Phenylpropenoyl Sucroses from Polygala tenuifolia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Compounds 1 to 3
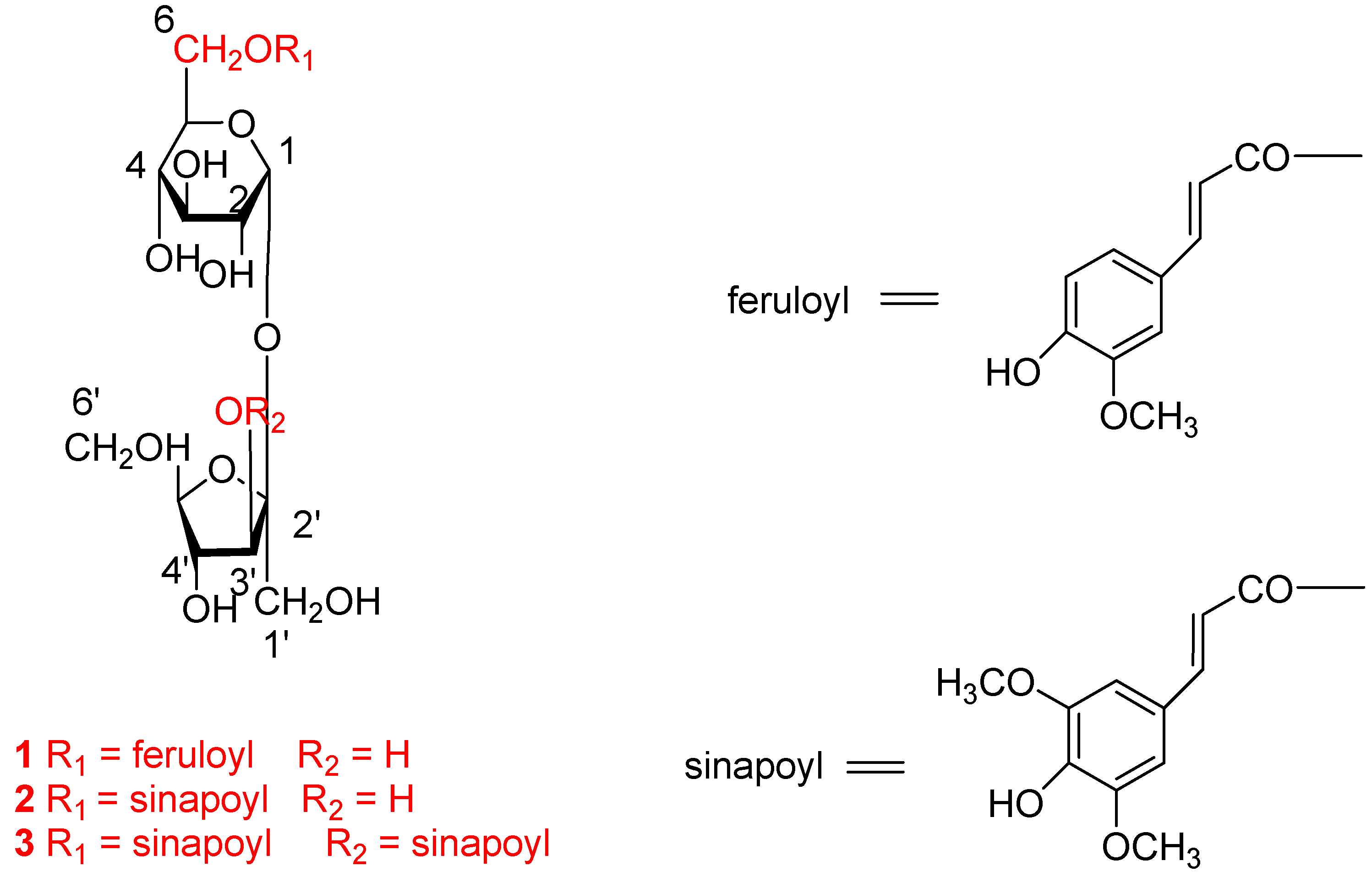
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| Glycone moiety | ||||||
| Glc-1 | 93.3 | 5.36 (d, 4) | 93.3 | 5.37 (d, 4) | 92.9 | 5.50 (d, 3.7) |
| 2 | 73.1 | 3.53 (d, 10.0, 4.0) | 73.1 | 3.51 (d, 10.0, 4.0) | 73.1 | 3.50 (m) |
| 3 | 74.9 | 3.69 (dd, 10.0, 9.0) | 75.0 | 3.75 (dd, 10.0, 9.5) | 75.0 | 3.69 (t, 9.3) |
| 4 | 71.2 | 3.38 (dd, 9.0, 9.0) | 71.2 | 3.34 (dd, 9.5, 9.0) | 71.9 | 3.38 (m) |
| 5 | 73.8 | 3.88 (m) | 74.5 | 3.89 (m) | 72.8 | 4.29 (m) |
| 6 | 62.3 | 3.77 (dd, 12.0, 4.5)3.81 (m) | 62.4 | 4.31 (dd, 12.0, 4.5)4.52 (m) | 65.6 | 4.48 (br d, 10.0)4.06 (m) |
| Fru-1 | 65.4 | 3.60 (d, 12.0) | 65.4 | 3.62 (d, 12.0) | 65.4 | 3.60 (d, 12.0) |
| 3.69 (d, 12.0) | 3.65 (d, 12.0) | 3.63 (d, 12.0) | ||||
| 2 | 104.4 | 104.8 | 104.8 | |||
| 3 | 79.7 | 5.40 (d, 8.0) | 79.6 | 5.46 (d, 8.0) | 79.6 | 5.58 (d, 7.5) |
| 4 | 74.8 | 4.33 (dd, 8.0, 7.5) | 74.5 | 4.07 (dd, 8.0, 7.5) | 74.4 | 4.51 (t, 8.0) |
| 5 | 84.1 | 3.88 (m) | 84.2 | 3.88 (m) | 84.2 | 4.10 (m) |
| 6 | 62.9 | 3.84 (m) | 62.9 | 3.89 (m) | 63.7 | 3.99 (m) |
| 3.84 (m) | 3.80 (m) | 4.20 (m) | ||||
| Aglycone moiety | ||||||
| R1-1 | 127.5 | 129.5 | 125.1 | |||
| 2 | 112.1 | 6.74 (d, 2.0) | 107.1 | 6.90 (s) | 106.8 | 7.12 (s) |
| 3 | 149.6 | 149.5 | 149.0 | |||
| 4 | 149.0 | 130.7 | 140.5 | |||
| 5 | 116.5 | 6.68 (d, 8.0) | 149.5 | 149.0 | ||
| 6 | 124.3 | 6.59 (dd, 8.0, 2.0) | 107.1 | 6.90 (s) | 106.8 | 7.12 (s) |
| 7 | 147.7 | 7.71 (d, 15.6) | 147.2 | 7.64 (d, 15.6) | 146.0 | 8.09 (d, 16.0) |
| 8 | 115.1 | 6.36 (d, 15.6) | 115.8 | 6.38 (d, 15.6) | 115.2 | 6.90 (d, 16.0) |
| 9 | 168.3 | 169.1 | 166.8 | |||
| -OCH3 | 56.6 | 3.86 (s) | 56.8 | 3.87 (s) | 56.4 | 3.80 (s) |
| R2-1 | 125.2 | |||||
| 2 | 106.9 | 7.12 (s) | ||||
| 3 | 149.2 | |||||
| 4 | 140.5 | |||||
| 5 | 149.2 | |||||
| 6 | 107.9 | 7.12 (s) | ||||
| 7 | 146.7 | 7.98 (d, 15.6) | ||||
| 8 | 115.6 | 6.67 (d, 15.6) | ||||
| 9 | 167.7 | |||||
| -OCH3 | 56.9 | 3.80 (s) | ||||
2.2. Untargeted Plasma Metabolite Profiling of Yuan zhi
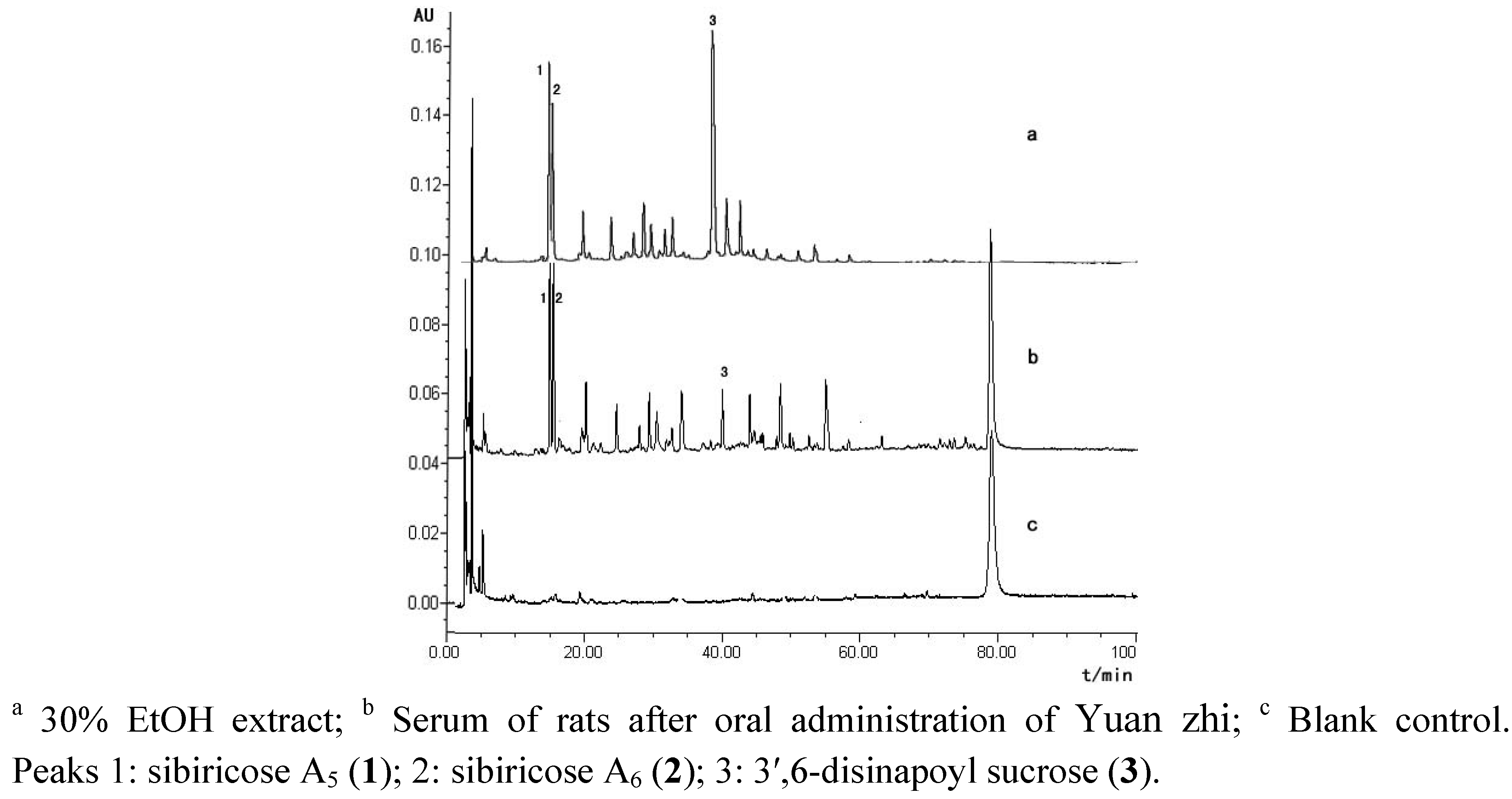
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. HPLC Analysis
3.5. Animal Experiments
Acknowledgements
References
- Kako, M.; Miura, T.; Nishiyama, Y.; Ichimaru, M.; Moriyasu, M.; Atsushi, K. Hypoglycemic activity of some triterpenoid glycosides. J. Nat. Prod. 1997, 60, 604–605. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, Y.; Kiyohara, H.; Susa, E.; Kato, T.; Nagamine, T.; Hagiwara, Y.; Tamura, S.; Yabe, T.; Aizawa, C.; et al. Onjisaponins, from the root of Polygala tenuifolia Willdenow, as effective adjuvants for nasal influenza and diphtheria-pertussis-tetanus vaccines. Vaccine 2001, 19, 4824–4834. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Ueno, T.; Kadoya, M.; Matsuda, H.; Yamahara, J.; Murakami, N. Bioactive saponins and glycosides. 1. Senegae radix. (1): E-senegasaponins a and b and Z-senegasaponins a and b, their inhibitory effect on alcohol absorption and hypoglycemic activity. Chem. Pharm. Bull. 1995, 43, 350–352. [Google Scholar] [CrossRef]
- Campos, R.O.P.; Santos, A.R.S.; Vaz, Z.R.; Pinheiro, T.R.; Pizzolatti, M.G.; Filho, V.C.; Monache, F.D.; Yunes, R.A.; Calixto, J.B. Antinociceptive properties of the hydroalcoholic extract and prelininary study of a xanthone isolated from Polygala cyparissias (Polygalaceae). Life Sci. 1997, 61, 1619–1630. [Google Scholar] [CrossRef]
- Yukinobu, I.; Shigefumi, T.; Mitsuo, T.; Humito, K.; Kouin, T.; Takuji, Y.; Masaki, A. Cognitive improving and cerebral protective effects of acylated oligosaccharides in Polygala tenuifolia. Biol. Pharm. Bull. 2004, 27, 1081–1085. [Google Scholar] [CrossRef]
- Miyase, T.; Iwata, Y.; Ueno, A. Tenuifolioses A-F, oligosaccharide multi-esters from the roots of Polygala Tenuifolia Willd. Chem. Pharm. Bull. 1991, 39, 3082–3084. [Google Scholar] [CrossRef]
- Kobayashi, S.; Miyase, T.; Noguchi, H. Polyphenolia glycosides and oligosaccharide multiesters from the roots of Polygala dalmaisiana. J. Nat. Prod. 2002, 65, 319–328. [Google Scholar] [CrossRef]
- Chang, H.T.; Tu, P.F. New oligosaccharide ester and xanthone C-glucosides from Polygala Telephioides. Helv. Chim. Acta 2007, 90, 944–950. [Google Scholar] [CrossRef]
- Zuo, F.; Zhou, Z.M.; Yan, M.Z.; Liu, M.L.; Xiong, Y.L.; Zhang, Q.; Song, H.Y.; Ye, W.H. Metabolism of constituents in Huangqin-Tang, a prescription in traditional Chinese medicine, by human intestinal flora. Biol. Pharm. Bull. 2002, 25, 558–563. [Google Scholar] [CrossRef]
- Song, R.; Xu, L.; Xu, F.G.; Li, Z.; Dong, H.J.; Tian, Y.; Zhang, Z.J. In vivo metabolism study of rhubarb decoction in rat using high-performance liquid chromatography with UV photodiode-array and mass-spectrometric detection: A strategy for systematic analysis of metabolites from traditional Chinese medicines in biological samples. J. Chromatogr. A 2010, 1217, 7144–7152. [Google Scholar]
- Tu, H.H.; Liu, P.; Mu, L.; Liao, H.B.; Xie, T.T.; Ma, L.H.; Liu, Y.M. Study on antidepressant components of sucrose ester from Polygala tenuifolia. Zhongguo Zhong Yao Za Zhi 2008, 33, 1278–1280. [Google Scholar]
- Miyase, T.; Ioguchi, H.; Chen, X.M. Sucrose ester and xanth one C-glycosides from the roots of Polygala sibirica. J. Nat. Prod. 1999, 62, 993–996. [Google Scholar] [CrossRef]
- Miyase, T.; Ueno, A. Sucrose derivatives from the roots of Polygala tenuifolia. Nat. Med. (Tokyo) 1993, 47, 267–278. [Google Scholar]
- Liu, P.; Hu, Y.; Guo, D.H.; Wang, D.X.; Tu, H.H.; Ma, L.; Xie, T.T.; Kong, L.Y. Potential antidepressant properties of radix polygalae (Yuanzhi). Phytomedicine 2010, 17, 794–799. [Google Scholar] [CrossRef]
- Yong, J.; Na, Z.; Zheng, C.; Peng, F.T. Fingerprint of polygala tenuifolia by high performance liquid chromatography. Yao Xue Xue Bao 2006, 41, 179–183. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
She, G.; Ba, Y.; Liu, Y.; Lv, H.; Wang, W.; Shi, R. Absorbable Phenylpropenoyl Sucroses from Polygala tenuifolia. Molecules 2011, 16, 5507-5513. https://doi.org/10.3390/molecules16075507
She G, Ba Y, Liu Y, Lv H, Wang W, Shi R. Absorbable Phenylpropenoyl Sucroses from Polygala tenuifolia. Molecules. 2011; 16(7):5507-5513. https://doi.org/10.3390/molecules16075507
Chicago/Turabian StyleShe, Gaimei, Yinying Ba, Yang Liu, Hang Lv, Wei Wang, and Renbing Shi. 2011. "Absorbable Phenylpropenoyl Sucroses from Polygala tenuifolia" Molecules 16, no. 7: 5507-5513. https://doi.org/10.3390/molecules16075507




