Anti-Migration Effects of Gekko Sulfated Glycopeptide on Human Hepatoma SMMC-7721 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Homogeneity Analysis
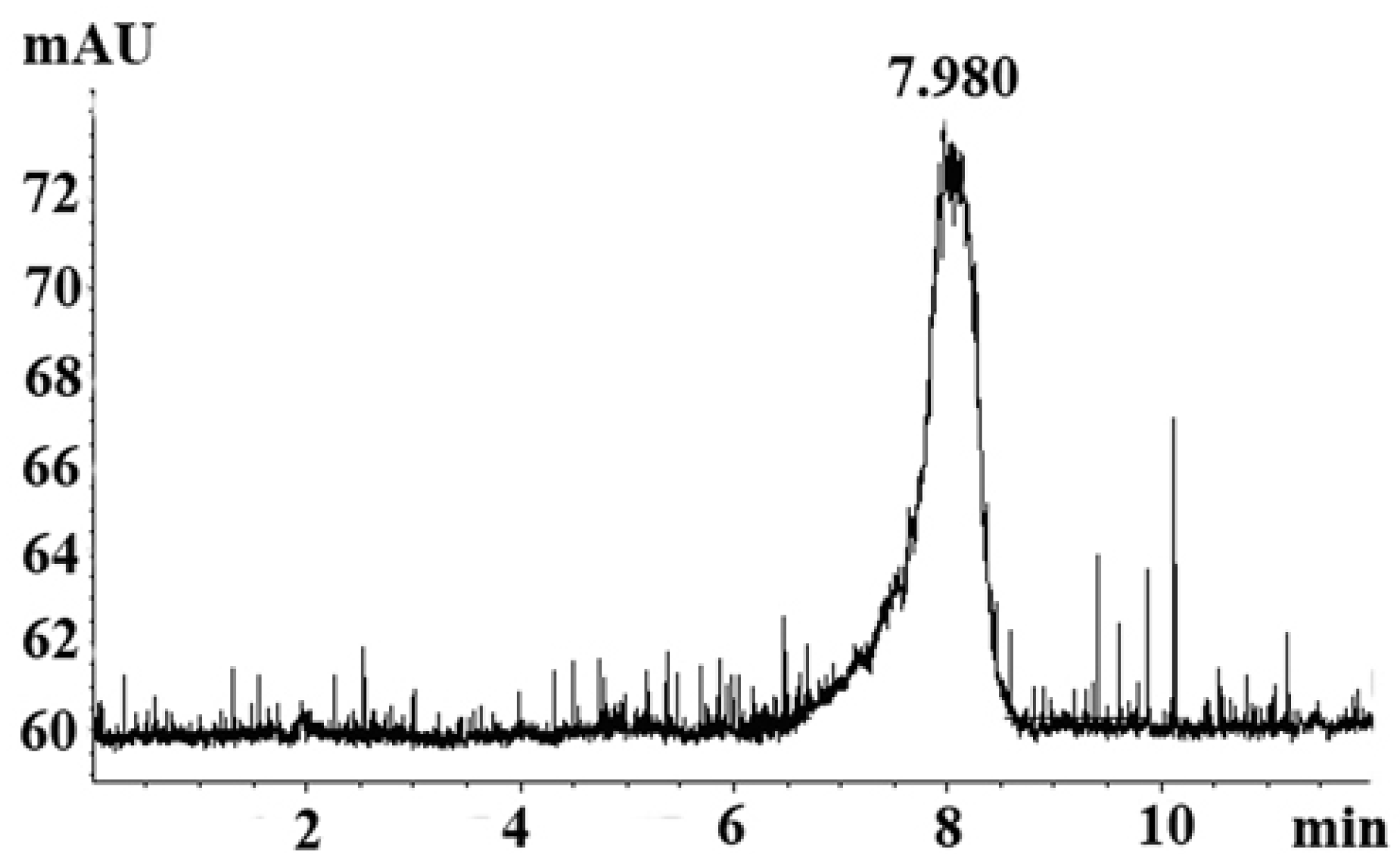
2.2. Fourier Transform Infrared Spectrum (FTIR) Analysis
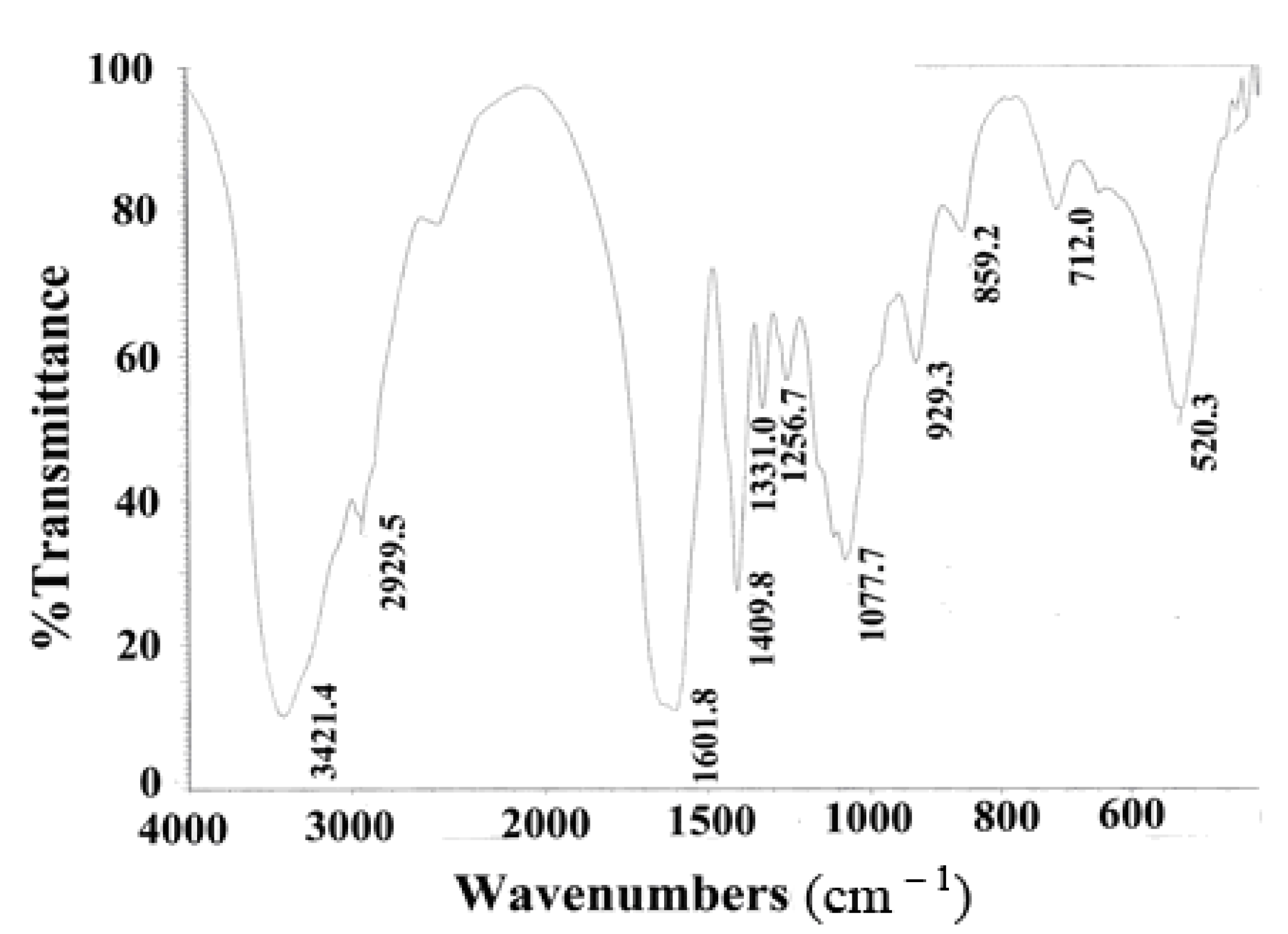
2.3. Amino Acid Composition and Glycopeptide Linkage Analysis
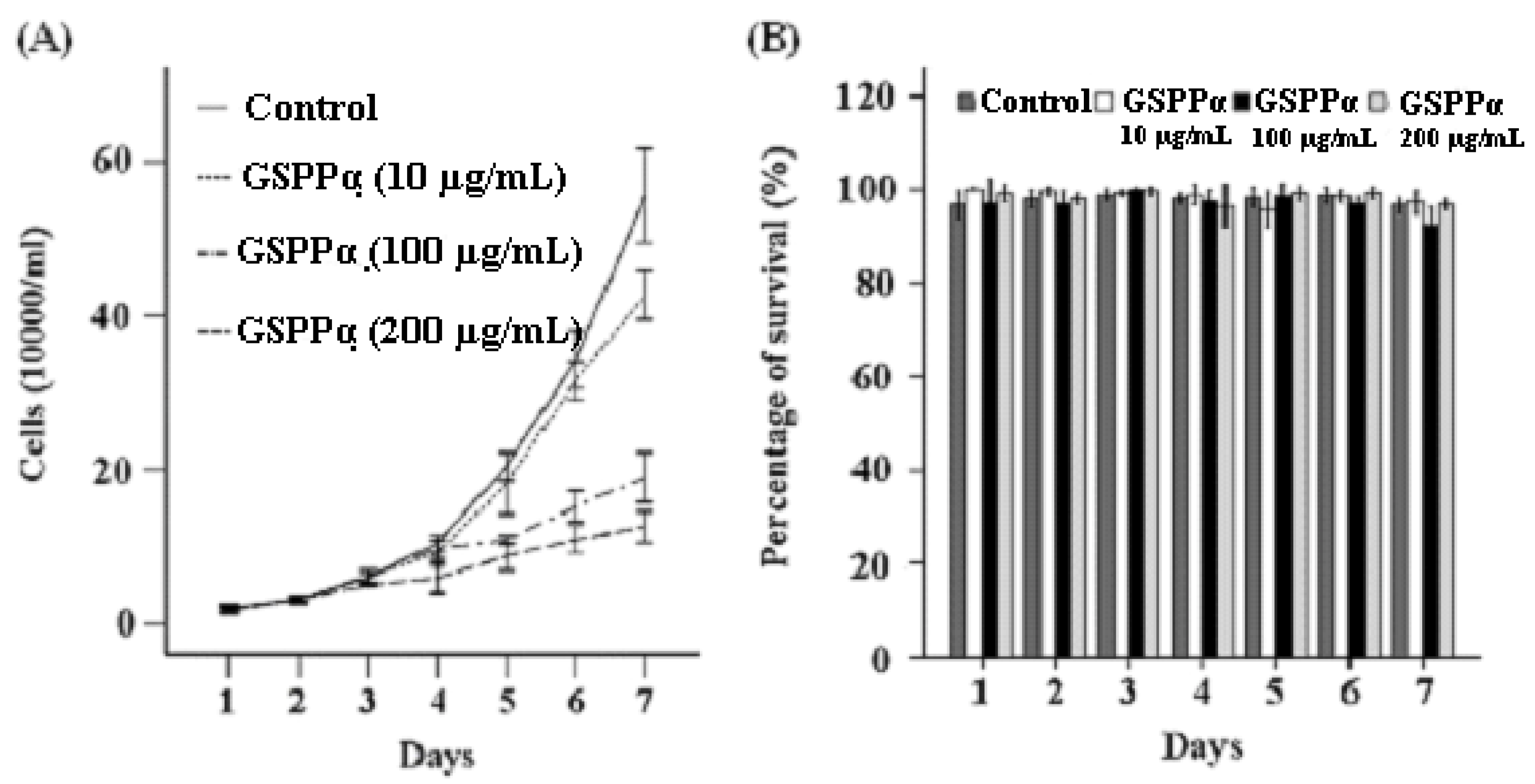
2.4. Effects of GSPP α on the Proliferation and Cell Cycle Distribution of SMMC-7721 Cells
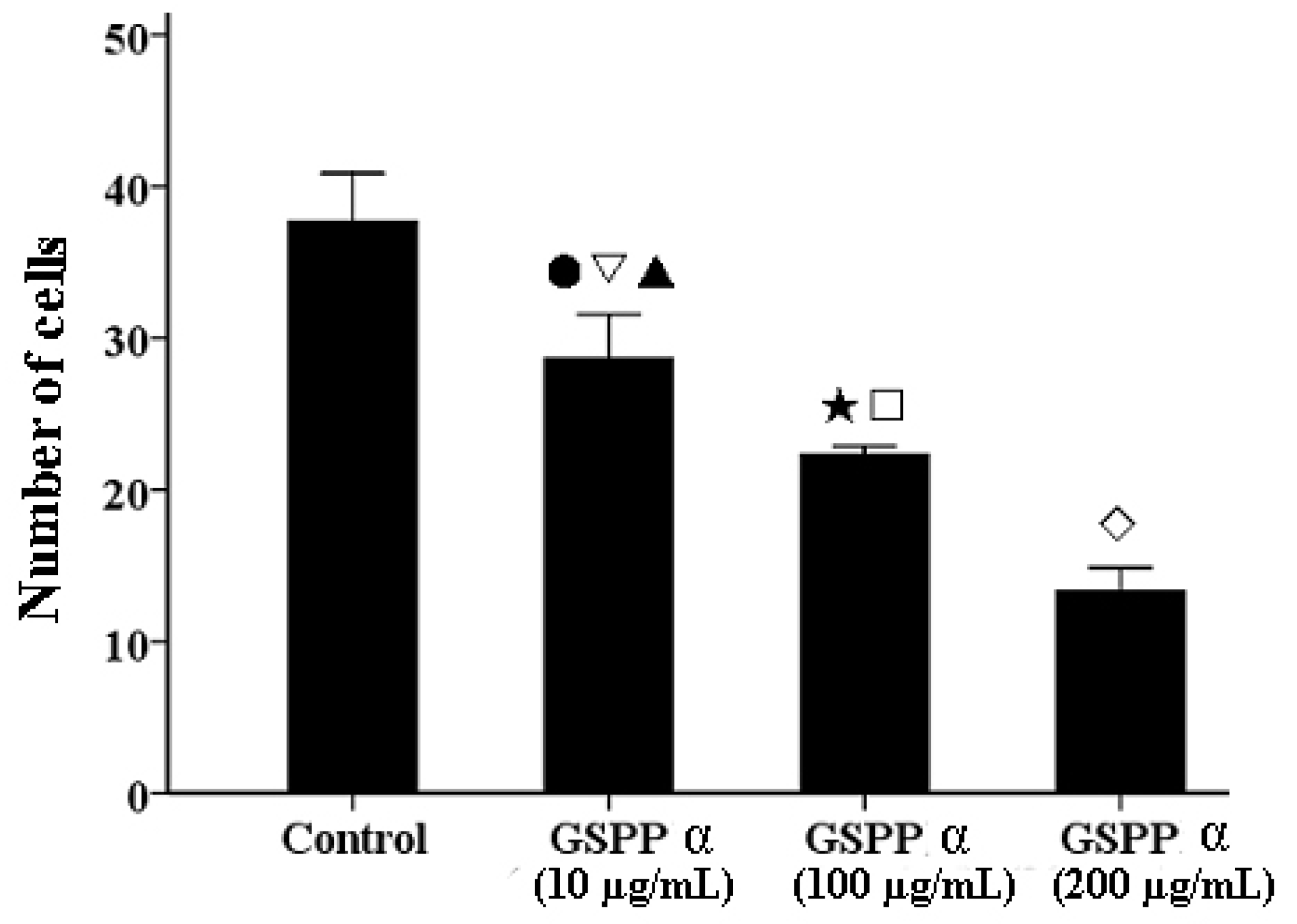
2.5. Effects of GSPP α on the Migration of SMMC-7721 Cells

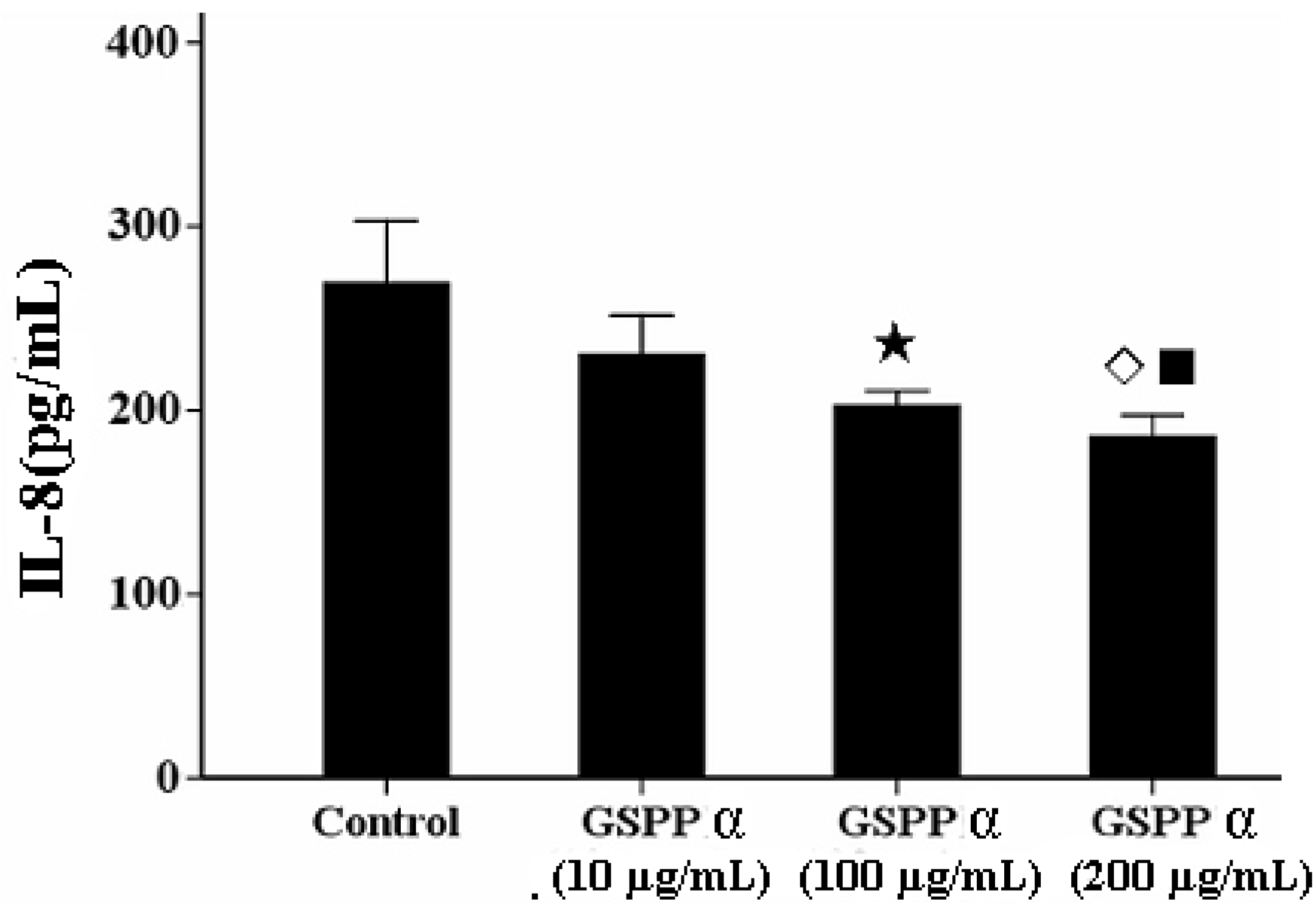
2. 6. Effects of GSPP α on The IL-8 Secretion from SMMC-7721 Cells
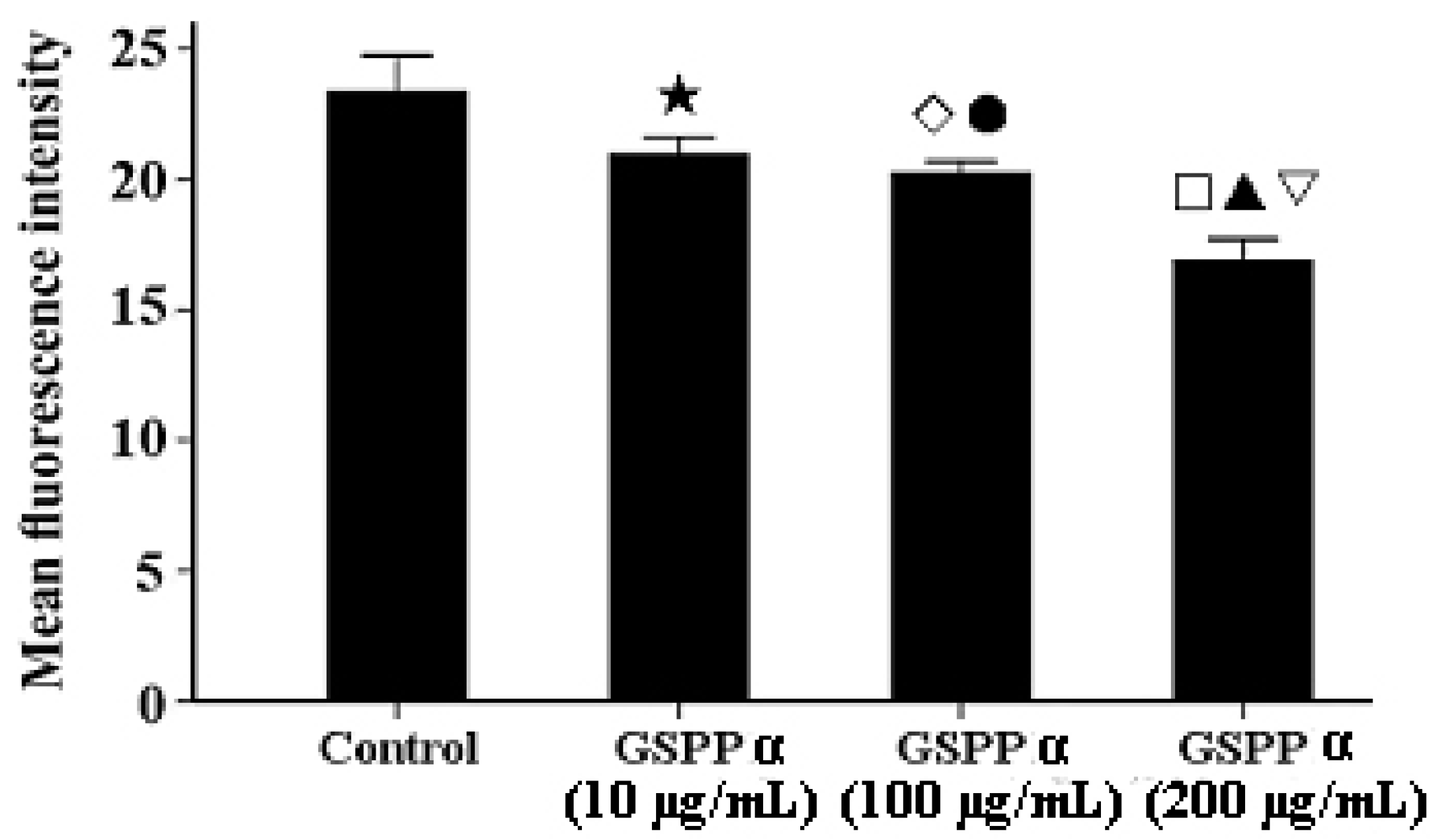
2.7. Effects of GSPP α on the Intracellular Calcium in SMMC-7721 Cells
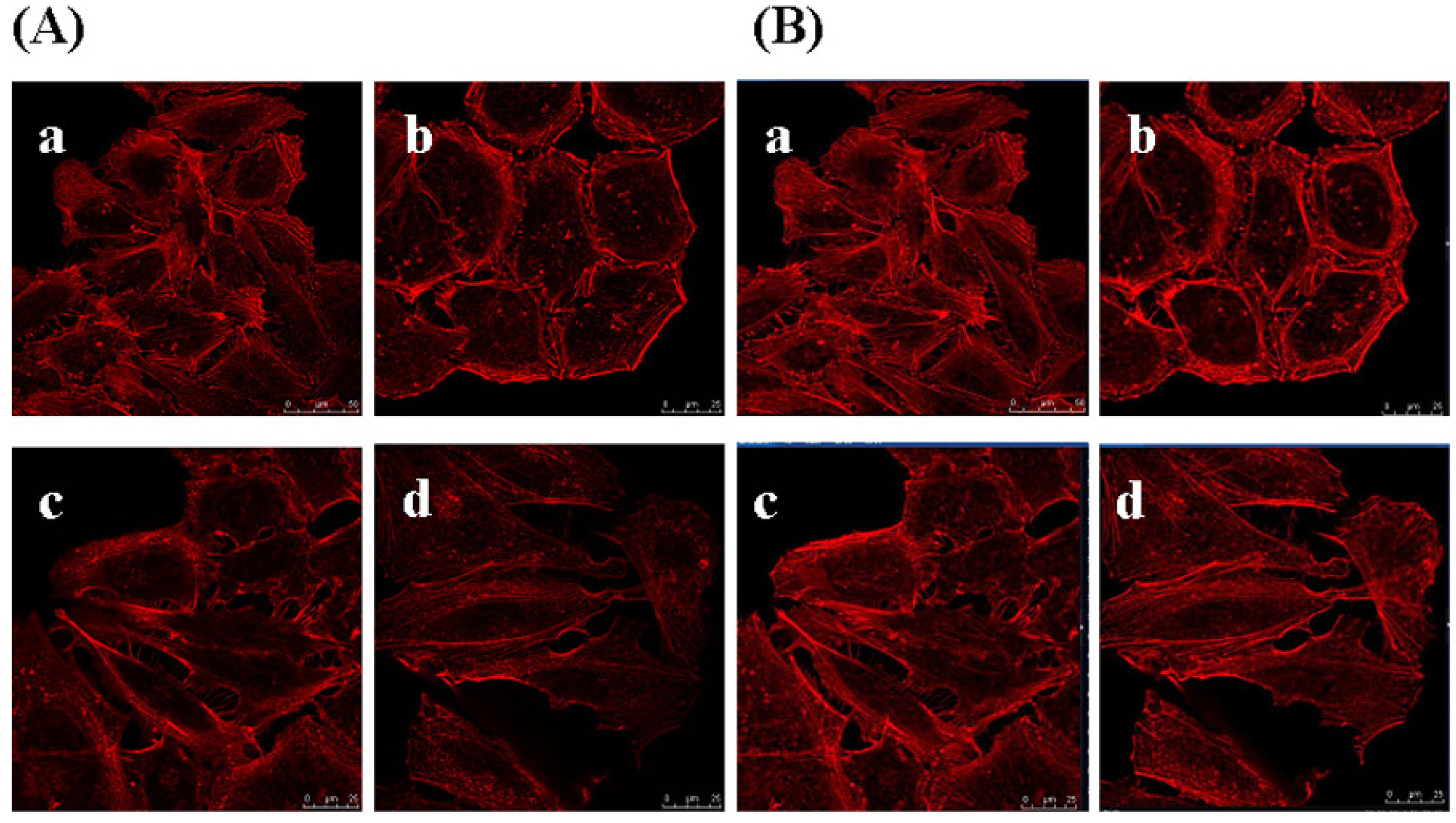
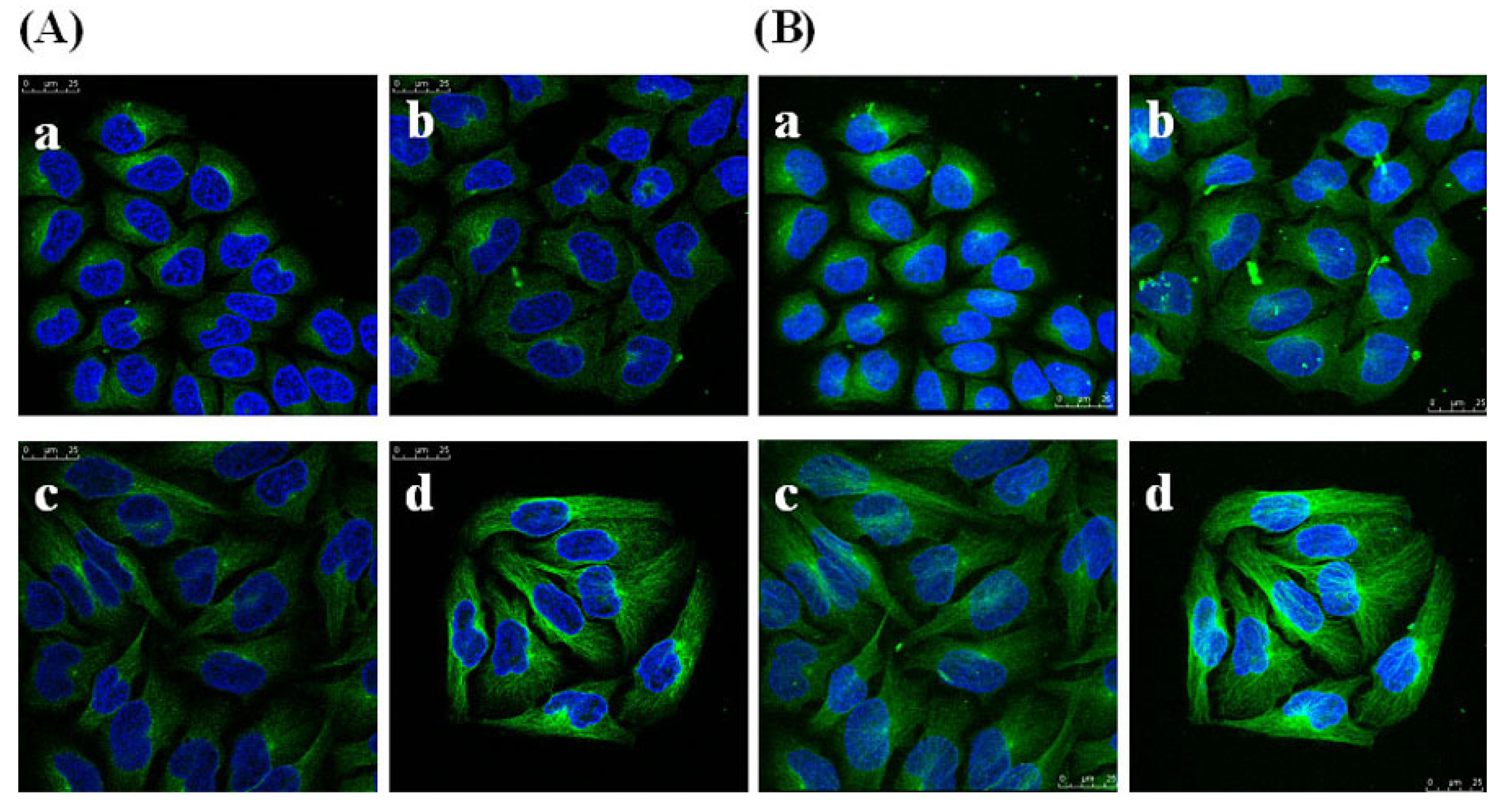
2.8. Effects of GSPP α on the Cytoskeleton in SMMC-7721 Cells
3. Experimental
3.1. Cell Culture and Reagents
3.2. Preparation of GSPP α
3.3. HPLC Analysis
3.4. General Analysis of GSPP α
3.5. β-Elimination Reaction
3.6. Trypan Blue Exclusion Assay and Flow Cytometry Analysis
3.7. Wound-Healing Assay and Transwell Assay
3.8. ELISA
3.9. Confocal Microscopy
3.10. Statistics and Data Analysis
4. Conclusions
Acknowledgements
References
- Hall, A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009, 28, 5–14. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk Sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Joyce, J.A.; Freeman, C.; Meyer-Morse, N.; Parish, C.R.; Hanahan, D. A functional heparan sulfatemimetic implicates both heparanase and heparan sulfate in tumor angiogenesis and invasion in a mouse model of multistage cance. Oncogene 2005, 24, 4037–4051. [Google Scholar]
- Miao, H.Q.; Elkin, M.; Aingorn, E.; Ishai-Michaeli, R.; Stein, C.A.; Vlodavsky, I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer 1999, 83, 424–431. [Google Scholar] [CrossRef]
- Parish, C.R.; Freeman, C.; Brown, K.J.; Francis, D.J.; Cowden, W.B. Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 1999, 59, 3433–3441. [Google Scholar]
- Wu, L.H.; Shi, B.Z.; Zhao, Q.L.; Wu, X.Z. Fucosylated glycan inhibition of human hepatocellular carcinoma cell migration through binding to chemokine receptors. Glycobiology 2010, 20, 215–223. [Google Scholar] [CrossRef]
- Chen, D.; Yao, W.J.; Zhang, X.L.; Han, X.Q.; Qu, X.Y.; Ka, W.B.; Sun, D.G.; Wu, X.Z.; Wen, Z.Y. Effects of Gekko sulfated polysaccharide-protein complex on human hepatoma SMMC-7721 cells: Inhibition of proliferation and migration. J. Ethnopharmacol. 2010, 127, 702–708. [Google Scholar] [CrossRef]
- Wu, X.Z.; Chen, D.; Xie, G.R. Effects of Gekko sulfated polysaccharide on the proliferation and differentiation of hepatic cancer cell line. Cell Biol. Int. 2006, 30, 659–664. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Q.; Chen, J.; Zeng, F. Solution properties of anti-tumor sulfated derivative of alpha-(1→3)-d-glucan from Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2000, 64, 2172–2178. [Google Scholar] [CrossRef]
- Wei, W.; Ryu, J.K.; Choi, H.B.; McLarnon, J.G. Expression and function of the P2X (7) receptor in rat C6 glioma cells. Cancer Lett. 2008, 260, 79–87. [Google Scholar] [CrossRef]
- Lang, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Signal processing in migrating T24 human bladder carcinoma cells: Role of the autocrine interleukin-8 loop. Int. J. Cancer 2002, 99, 673–680. [Google Scholar] [CrossRef]
- Garib, V.; Lang, K.; Niggemann, B.; Zanker, K.S.; Brandt, L.; Dittmar, T. Propofol-induced calcium signaling and actin reorganization within breast carcinoma cells. Eur. J. Anaesthesiol. 2005, 22, 609–615. [Google Scholar]
- Jiang, P.; Enomoto, A.; Takahashi, M. Cell biology of the movement of breast cancer cells: Intracellular signalling and the actin cytoskeleton. Cancer Lett. 2009, 284, 122–130. [Google Scholar] [CrossRef]
- Feldner, J.C.; Brandt, B.H. Cancer cell motility-on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp. Cell Res. 2002, 272, 93–108. [Google Scholar] [CrossRef]
- Ballestrem, C.; Wehrle-Haller, B.; Hinz, B.; Imhof, B.A. Actin-dependent lamellipodia formation and microtubule-dependent tail retraction control-directed cell migration. Mol. Biol. Cell 2000, 11, 2999–3012. [Google Scholar]
- Sentandreu, R.; Northcote, D.H. The Structure of a glycopeptide isolated from the yeast cell wall. Biochem. J. 1968, 109, 419–432. [Google Scholar]
- Sevage, M.G. Mechanism of the respiration of Pneumococci II. Biochem. J. 1934, 273, 419–429. [Google Scholar]
- Zhao, H.; Liu, H.; Chen, Y.; Xin, X.; Li, J.; Hou, Y.; Zhang, Z.; Zhang, X.; Xie, C.; Geng, M.; Ding, J. Oligomannurarate sulfate, a novel heparanase inhibitor simultaneously targeting basic fibroblast growth factor, combats tumor angiogenesis and metastasis. Cancer Res. 2006, 66, 8779–8787. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar]
- Yang, C.; Zeisberg, M.; Lively, J.C.; Nyberg, P.; Afdhal, N.; Kalluri, R. Integrin alpha1beta1 and alpha2beta1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res. 2003, 63, 8312–8317. [Google Scholar]
- Yao, W.; Gu, L.; Sun, D.; Ka, W.; Wen, Z.; Chien, S. Wild type p53 gene causes reorganization of cytoskeleton and, therefore, the impaired deformability and difficult migration of murine erythroleukemia cells. Cell Motil. Cytoskeleton 2003, 56, 1–12. [Google Scholar] [CrossRef]
- Samples Availability: Samples Not Available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, X.-Z.; Chen, D.; Han, X.-Q. Anti-Migration Effects of Gekko Sulfated Glycopeptide on Human Hepatoma SMMC-7721 Cells. Molecules 2011, 16, 4958-4970. https://doi.org/10.3390/molecules16064958
Wu X-Z, Chen D, Han X-Q. Anti-Migration Effects of Gekko Sulfated Glycopeptide on Human Hepatoma SMMC-7721 Cells. Molecules. 2011; 16(6):4958-4970. https://doi.org/10.3390/molecules16064958
Chicago/Turabian StyleWu, Xiong-Zhi, Dan Chen, and Xiao-Qiang Han. 2011. "Anti-Migration Effects of Gekko Sulfated Glycopeptide on Human Hepatoma SMMC-7721 Cells" Molecules 16, no. 6: 4958-4970. https://doi.org/10.3390/molecules16064958



