Abstract
In this study, a series of twenty-two 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides and ten 4-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides is described. The compounds were analyzed using RP-HPLC to determine lipophilicity. Primary in vitro screening of the synthesized compounds was performed against mycobacterial, bacterial and fungal strains. They were also evaluated for their activity related to the inhibition of photosynthetic electron transport (PET) in spinach (Spinacia oleracea L.) chloroplasts. The compounds showed biological activity comparable with or higher than the standards isoniazid, fluconazole, penicillin G or ciprofloxacin. For all the compounds, the relationships between the lipophilicity and the chemical structure of the studied compounds as well as their structure-activity relationships are discussed.
1. Introduction
Salicylanilides (2-hydroxy-N-phenylbenzamides) are an important class of aromatic compounds with a wide range of pharmacological activities. A number of them show antibacterial [1,2,3], antimycobacterial [4,5,6] and antifungal [4,7] as well as anti-inflammatory [8] or antineoplastic activities [9,10,11]. In spite of their promise as potential drugs, certain physico-chemical properties of salicylanilides, for example low solubility, prevented their widespread use in clinical practice. Thus, improvement of the physical-chemical properties of salicylanilides is an interesting and vital area of research.
We have focused our efforts on design and synthesis of original O-substituted derivates of salicylanilides. Promising results of antimycobacterial evaluation of some salicylanilides acetates [4] inspired us to design more sophisticated derivates of salicylanilides – amino acid esters. The design strategy of these potential antimicrobial active agents was mainly oriented towards the synthesis of a prodrug form of most antitubercular active salicylanilides by attaching an amino acid. The phenolic hydroxyl group of the most antitubercular active salicylanilides was esterified by several N-benzyloxy-carbonyl α-amino acids. More lipophilic amino acids such as glycine, (R and S)-alanine, (R and S)-valine and (R and S)-phenylalanine were chosen. The prepared N-protected esters were tested for their antifungal and antimycobacterial activity; promising results were published recently [5,6,7]. Subsequent processing using standard reactions yielded unexpected products (4-chloro- and 5-chloro-N-salicylamides for detailed structure see Scheme 1) that were described, including proposed reaction mechanism, and published [12,13,14].
It was expected that chloro-2-hydroxy-N-(arylalkyl)benzamides, compounds with a special skeleton originating from biologically active salicylanilides, would have interesting biological properties. A series of these compounds was prepared, and most of them were tested for their antimycobacterial, antifungal and antibacterial activity. As the presence of an amide (-NHCO-) group in acylanilides, phenylcarbamates, ureas, etc., is characteristic of a number of herbicides acting as photosynthesis inhibitors [15,16,17,18,19,20,21,22,23,24,25], the compounds were also evaluated for their photosynthesis-inhibiting activity (the inhibition of photosynthetic electron transport) in spinach chloroplasts (Spinacia oleracea L.). These compounds bind reversibly to photosystem II (PS II), a membrane-protein complex in the thylakoid membranes, which catalyses oxidation of water and reduction of plastoquinone [15], and thereby inhibit photosynthesis [26,27,28,29,30]. For example, in the presence of R' substituted salicylanilides the decreased intensity of the fluorescence emission band at 686 nm (belonging to the chlorophyll-protein complexes mainly in PS II [31]) suggested PS II as the site of action of the studied inhibitors [24].
Many low molecular weight drugs cross biological membranes through passive transport, which strongly depends on their lipophilicity, therefore this physico-chemical parameter of the prepared compounds was determined by means of the RP-HPLC. Relationships between the structure and in vitro antimicrobial activities or/and inhibitory activity related to inhibition of photosynthetic electron transport (PET) in spinach chloroplasts of the new compounds are discussed.
2. Results and Discussion
2.1. Chemistry
Preparation of substituted 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides 8a-v and 4-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides 9a-j was carried out by two-step synthesis with a subsequent rearrangement (Scheme 1). The first step was dicyclocarbodiimide (DCC) mediated esterification of N-protected amino acids 3 (benzyloxycarbonyl group as protection) with selected antimicrobial active salicylanilides 1 and 2 in dry dimethylformamide (DMF). Esters 4 and 5 were obtained optically pure in high yields [5,7].
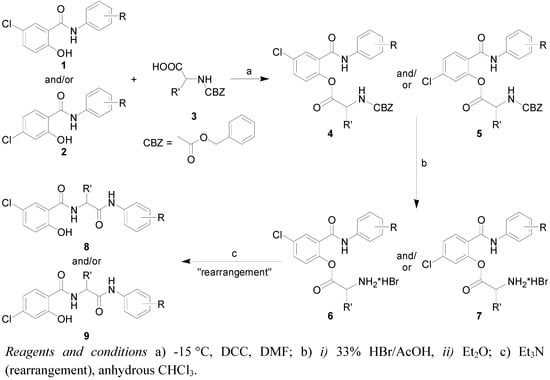
Scheme 1.
Synthetic pathway for preparation of target substituted 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides 8a-8v and 4-chloro-2-hydroxy-N-[2-(aryl-amino)-1-alkyl-2-oxoethyl]benzamides 9a-9j.
Acidolysis (33% HBr in acetic acid) was used as a method for N-deprotection and this method gave appropriate hydrobromide salts 6 and 7 in almost quantitative yields. Subsequent amino group liberation by triethylamine in anhydrous chloroform yielded unexpected rearranged products 8 and 9 [12,13]. This methodology is general and is not dependent on the substitution of the aromatic salicylanilide ring as well as on the amino acid type. A small combinatorial library of discussed compounds 8 and 9 was recently synthesized and published [22]. The compounds could be divided into six groups based on their chemical structure: Group 1 includes 5-Cl/3-Cl substituted diamides 8a-8g; Group 2 contains 5-Cl/4-Cl derivatives 8h-8l; Group 3 includes 5-Cl/3,4-Cl derivatives 8m-8r; Group 4 includes 5-Cl/4-Br derivatives 8s-8v; Group 5 contains 4-Cl/4-Cl derivatives 9a-9f; and Group 6 is composed of 4-Cl/4-X diamides 9g-9j.
2.2. Lipophilicity
Lipophilicity is a property that has a major effect on absorption, distribution, metabolism, excretion and toxicity properties as well as pharmacological activity, because drugs cross biological membranes through passive transport, which strongly depends on their lipophilicity. Lipophilicity has been studied and applied as an important drug property for decades [32].
Hydrophobicities (log P/Clog P) of compounds 8 and 9 were calculated using two commercially available programs (ChemOffice Ultra 10.0 and ACD/LogP) and measured by means of RP-HPLC determination of capacity factors k with subsequent calculation of log k. The procedure was performed under isocratic conditions with methanol as an organic modifier in the mobile phase using an end-capped non-polar C18 stationary RP column. The results are shown in Table 1 and illustrated in Figure 1.
Both used programs did not resolve lipophilicity parameters within the series of enantiomers, as expected. The program ChemOffice did not resolve various lipophilicity values of individual positional isomers, in particular, the same log P/Clog P data were calculated for compounds 8a-8g from Group 1, 8h-8l from Group 2, 9h-9f from Group 5, 8s-8v from Group 4 and compound 4-Cl/4-Br (9g) from Group 6.
The results obtained with all the compounds show that the experimentally-determined lipophilicities (log k) of the discussed compounds are in accordance with the calculated values of compounds 8 and 9 as shown in Figure 1. Compounds series 9 substituted by chlorine in C(4) position showed higher lipophilicity than compounds series 8 with C(5) chlorine substitution, although program ACD/LogP showed an opposite trend. According to the program ACD/LogP, compounds 5a-5g substituted by chlorine in C'(3) position showed higher lipophilicity than compounds 5h-5l with C'(4) chlorine substitution, but experimental log k for C'(4) chlorine substitution is higher. As expected, the lipophilicity of the compounds substituted in the anilide part of the molecule increases as follows: OCH3 < CH3 < Cl < Br < CF3 < di-Cl, although program ACD/LogP calculates higher log P for Br derivative 9g than CF3 substituted compound 9i. The lipophilicity factor log k as well as calculated log P (ACD/LogP) and log P/Clog P values (ChemOffice) increase, depending on amino acid type (glycine, alanine, valine and phenylalanine), that is, the lipophilicity of the compounds substituted in R3 increases as follows: H < CH3 < CH(CH3)2 < CH2C6H5.
Log k data specify lipophilicity within this series of the discussed compounds, and all the chiral compounds were measured several times with the same results. The determined differences in log k parameters for individual R/S-enantiomers cannot be explained on the basis of the results presented here.

Table 1.
Comparison of calculated lipophilicities (log P/Clog P) with determined log k values, Hammett's parameter (σ) and bulk parameter (MR, reflecting bulkiness).
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | R1 | R2 | R3 | log k | log P/Clog P ChemOffice | log P ACD/LogP | σR2[33] | MRR3[33] | |||
| GROUP 1 | 8a | 5-Cl | 3-Cl | H | 0.2439 | 2.42 / 4.33156 | 4.36 ± 0.47 | 0.373 | 0 | ||
| 8b | 5-Cl | 3-Cl | (S)-CH3 | 0.4166 | 2.91 / 4.64056 | 4.71 ± 0.48 | 0.373 | 4.7 | |||
| 8c | 5-Cl | 3-Cl | (R)-CH3 | 0.4153 | 2.91 / 4.64056 | 4.71 ± 0.48 | 0.373 | 4.7 | |||
| 8d | 5-Cl | 3-Cl | (S)-CH(CH3)2 | 0.6124 | 3.80 / 5.56856 | 5.59 ± 0.48 | 0.373 | 14.0 | |||
| 8e | 5-Cl | 3-Cl | (R)-CH(CH3)2 | 0.6114 | 3.80 / 5.56856 | 5.59 ± 0.48 | 0.373 | 14.0 | |||
| 8f | 5-Cl | 3-Cl | (S)-CH2C6H5 | 0.7098 | 4.58 / 6.05856 | 6.64 ± 0.49 | 0.373 | 29.0 | |||
| 8g | 5-Cl | 3-Cl | (R)-CH2C6H5 | 0.7066 | 4.58 / 6.05856 | 6.64 ± 0.49 | 0.373 | 29.0 | |||
| GROUP 2 | 8h | 5-Cl | 4-Cl | (R)-CH3 | 0.4308 | 2.91 / 4.64056 | 4.64 ± 0.47 | 0.227 | 4.7 | ||
| 8i | 5-Cl | 4-Cl | (S)-CH(CH3)2 | 0.6371 | 3.80 / 5.56856 | 5.52 ± 0.48 | 0.227 | 14.0 | |||
| 8j | 5-Cl | 4-Cl | (R)-CH(CH3)2 | 0.6125 | 3.80 / 5.56856 | 5.52 ± 0.48 | 0.227 | 14.0 | |||
| 8k | 5-Cl | 4-Cl | (S)-CH2C6H5 | 0.7394 | 4.58 / 6.05856 | 6.57 ± 0.49 | 0.227 | 29.0 | |||
| 8l | 5-Cl | 4-Cl | (R)-CH2C6H5 | 0.7329 | 4.58 / 6.05856 | 6.57 ± 0.49 | 0.227 | 29.0 | |||
| GROUP 3 | 8m | 5-Cl | 3,4-Cl | H | 0.4971 | 2.98 / 5.01472 | 5.20 ± 0.49 | 0.600 | 0 | ||
| 8n | 5-Cl | 3,4-Cl | (S)-CH3 | 0.6698 | 3.47 / 5.32372 | 5.55 ± 0.50 | 0.600 | 4.7 | |||
| 8o | 5-Cl | 3,4-Cl | (S)-CH(CH3)2 | 0.8623 | 4.35 / 6.25172 | 6.43 ± 0.50 | 0.600 | 14.0 | |||
| 8p | 5-Cl | 3,4-Cl | (R)-CH(CH3)2 | 0.8614 | 4.35 / 6.25172 | 6.43 ± 0.50 | 0.600 | 14.0 | |||
| 8q | 5-Cl | 3,4-Cl | (S)-CH2C6H5 | 0.9485 | 5.14 / 6.74172 | 7.48 ± 0.51 | 0.600 | 29.0 | |||
| 8r | 5-Cl | 3,4-Cl | (R)-CH2C6H5 | 0.9408 | 5.14 / 6.74172 | 7.48 ± 0.51 | 0.600 | 29.0 | |||
| GROUP 4 | 8s | 5-Cl | 4-Br | (S)-CH(CH3)2 | 0.6709 | 4.07 / 5.71856 | 5.76 ± 0.54 | 0.232 | 14.0 | ||
| 8t | 5-Cl | 4-Br | (R)-CH(CH3)2 | 0.6646 | 4.07 / 5.71856 | 5.76 ± 0.54 | 0.232 | 14.0 | |||
| 8u | 5-Cl | 4-Br | (S)-CH2C6H5 | 0.7779 | 4.86 / 6.20856 | 6.81 ± 0.55 | 0.232 | 29.0 | |||
| 8v | 5-Cl | 4-Br | (R)-CH2C6H5 | 0.7673 | 4.86 / 6.20856 | 6.81 ± 0.55 | 0.232 | 29.0 | |||
| GROUP 5 | 9a | 4-Cl | 4-Cl | (S)-CH3 | 0.4934 | 2.91 / 4.64056 | 4.54 ± 0.47 | 0.227 | 4.7 | ||
| 9b | 4-Cl | 4-Cl | (R)-CH3 | 0.4921 | 2.91 / 4.64056 | 4.54 ± 0.47 | 0.227 | 4.7 | |||
| 9c | 4-Cl | 4-Cl | (S)-CH(CH3)2 | 0.6839 | 3.80 / 5.56856 | 5.41 ± 0.48 | 0.227 | 14.0 | |||
| 9d | 4-Cl | 4-Cl | (R)-CH(CH3)2 | 0.6832 | 3.80 / 5.56856 | 5.41 ± 0.48 | 0.227 | 14.0 | |||
| 9e | 4-Cl | 4-Cl | (S)-CH2C6H5 | 0.7540 | 4.58 / 6.05856 | 6.47 ± 0.49 | 0.227 | 29.0 | |||
| 9f | 4-Cl | 4-Cl | (R)-CH2C6H5 | 0.7479 | 4.58 / 6.05856 | 6.47 ± 0.49 | 0.227 | 29.0 | |||
| GROUP 6 | 9g | 4-Cl | 4-Br | (R)-CH(CH3)2 | 0.7353 | 4.07 / 5.71856 | 5.65 ± 0.54 | 0.232 | 14.0 | ||
| 9h | 4-Cl | 4-CF3 | (S)-CH(CH3)2 | 0.7892 | 4.16 / 5.93176 | 5.46 ± 0.51 | 0.740 | 14.0 | |||
| 9i | 4-Cl | 4-CH3 | (S)-CH(CH3)2 | 0.5902 | 3.72 / 5.09696 | 4.91 ± 0.46 | -0.170 | 14.0 | |||
| 9j | 4-Cl | 4-OCH3 | (S)-CH(CH3)2 | 0.3623 | 3.11 / 4.67336 | 4.46 ± 0.48 | -0.270 | 14.0 | |||
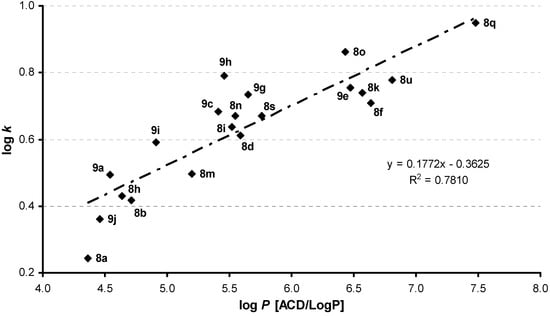
Figure 1.
Relationship between calculated log P data (ACD/LogP) and experimentally found log k values. If both enantiomers were prepared, only (S)-enantiomers are illustrated due to similar log k values.
2.3. Biological activities
The compounds showed a wide range of biological activities, and some noteworthy structure-activity relationships were observed. All the results are shown in Table 2. Generally, all the discussed compounds (especially compounds 8b, 8q, 8r) exhibited lower solubility in comparison with the initial esters [5,7].
2.3.1. Antimycobacterial screening
The discussed amides were tested against three Mycobacteria strains. According to the results of in vitro evaluation (see Table 2), all compounds demonstrated noteworthy effects. Activities of some benzamides, especially against M. avium and M. kansasii, considerably exceeded the activity of isoniazid (INH) used as an internal standard. The discussed compounds can be divided according to the activities against individual Mycobacteria strains, and structure-activity relationships within Group 1, Group 2 and Group 4 can be discussed. Compound 8b showed the lowest activity due to precipitation in the testing medium, contrary to its (R)-enantiomer 8c. Also compounds 8q and 8r precipitated from testing medium, therefore 8b, 8q and 8r were excluded from the below SAR discussion.
It can be concluded that compound N-{(1R)-1-benzyl-2-[(4-chlorophenyl)amino]-2-oxoethyl}-5-chloro-2-hydroxybenzamide (8l) and N-{(1R)-1-benzyl-2-[(4-chlorophenyl)amino]-2-oxoethyl}-4-chloro-2-hydroxybenzamide (9f) showed the highest antitubercular/antimycobacterial activity, see Table 2.

Table 2.
Antimycobacterial activity (MIC/IC90) of compounds in comparison with isoniazid (INH) standard; in vitro antifungal and antibacterial activity (MIC/IC50 or IC90) of compounds compared with fluconazole (FLU), penicillin G (PEN) and ciprofloxacin (CPF) standards; and IC50 values of discussed compounds related to photosynthetic electron transport (PET) inhibition in spinach chloroplasts in comparison with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) standard. (MIC-minimum inhibitory concentration, ND-not determined, MTB-Mycobacterium tuberculosis, MA-M. avium, MK-M.kansasii, CT-Candida tropicalis, CK-C. krusei, TM-Trichophyton mentagrophytes, SA-S. aureus, MRSA-methicilin resistant Staphylococcus aureus, SE-S. epidermidis, EF-Enterococcus sp.)
| Comp. | MIC [µmol/L] | PET IC50 [μmol/L] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTB a | MA a | MK a | CT b | CK b | TM c | SA d | MRSA d | SE d | EF d | |||
| 14h | 14h | 14h | 24h | 24h | 72h | 24h | 24h | 24h | 24h | |||
| 21h | 21h | 21h | 48h | 48h | 120h | 48h | 48h | 48h | 48h | |||
| GROUP 1 | 8a | 125 | 62.5 | 250 | 125 | 125 | 125 | >250 | >250 | >250 | >250 | 378.6 |
| 125 | 62.5 | 250 | 125 | 125 | 125 | >250 | >250 | >250 | >250 | |||
| 8be | 250 | 500 | 500 | >125 | >125 | 125 | >250 | >250 | >250 | >250 | 41.7 | |
| >500 | >500 | >500 | >125 | >125 | 125 | >250 | >250 | >250 | >250 | |||
| 8c | 62.5 | 125 | 62.5 | 31.25 | 31.25 | 0.49 | >500 | >500 | >500 | >500 | ND | |
| 125 | 250 | 62.5 | 125 | 62.5 | 0.49 | >500 | >500 | >500 | >500 | |||
| 8d | 32 | 125 | 62.5 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 13.8 | |
| 62.5 | 125 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8e | 32 | 62.5 | 62.5 | 15.62 | 3.9 | 125 | 15.62 | 31.25 | 31.25 | 62.5 | 20.4 | |
| 62.5 | 125 | 125 | 62.5 | 7.81 | 125 | 15.62 | 31.25 | 31.25 | 250 | |||
| 8f | 62.5 | 125 | 62.5 | 31.25 | 1.95 | 125 | >500 | >500 | >500 | >500 | 12.3 | |
| 125 | 250 | 125 | 125 | 3.9 | 125 | >500 | >500 | >500 | >500 | |||
| 8g | 62.5 | 32 | 62.5 | 31.25 | 1.95 | 125 | >500 | >500 | >500 | >500 | ND | |
| 62.5 | 32 | 62.5 | 125 | 3.9 | 125 | >500 | >500 | >500 | >500 | |||
| GROUP 2 | 8h | 8 | 125 | 125 | >125 | 125 | >125 | >500 | >500 | >500 | >500 | ND |
| 16 | 125 | 250 | >125 | 125 | >125 | >500 | >500 | >500 | >500 | |||
| 8i | 32 | 62.5 | 62.5 | >125 | >125 | >125 | 7.81 | 15.62 | 31.25 | 31.25 | ND | |
| 32 | 62.5 | 62.5 | >125 | >125 | >125 | 15.62 | 15.62 | 250 | 150 | |||
| 8j | 32 | 62.5 | 62.5 | >125 | 62.5 | 62.5 | 7.81 | 7.81 | 31.25 | 250 | ND | |
| 32 | 62.5 | 62.5 | >125 | 125 | 62.5 | 7.81 | 7.81 | 125 | 500 | |||
| 8k | 16 | 32 | 32 | 125 | 125 | 125 | >500 | >500 | >500 | >500 | 29.8 | |
| 16 | 32 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8l | 16 | 32 | 32 | 125 | 125 | 125 | >125 | >125 | >125 | >125 | 33.7 | |
| 16 | 32 | 32 | >125 | >125 | 125 | >125 | >125 | >125 | >125 | |||
| GROUP 3 | 8m | 125 | 62.5 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | ND |
| 500 | 500 | 500 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8n | 32 | 32 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| 32 | 62.5 | 500 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8o | 32 | 62.5 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| 32 | 125 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8p | 16 | 62.5 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| 32 | 125 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8qe | 125 | 125 | 250 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | 74.2 | |
| >1000 | >1000 | >1000 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 8re | 125 | 125 | 250 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | ND | |
| >1000 | >1000 | >1000 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| GROUP 4 | 8s | 62.5 | 62.5 | 62.5 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 26.2 |
| 62.5 | 62.5 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8t | 62.5 | 62.5 | 62.5 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | ND | |
| 62.5 | 62.5 | 125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 8u | 32 | 250 | 62.5 | 500 | 500 | 500 | >125 | >125 | >125 | >125 | 31.2 | |
| 32 | 250 | 125 | >500 | >500 | >500 | >125 | >125 | >125 | >125 | |||
| 8v | 32 | 250 | 32 | 500 | 500 | 500 | 3.9 | 7.81 | 62.5 | 62.5 | ND | |
| 32 | 250 | 32 | >500 | >500 | >500 | 15.62 | 15.62 | 125 | 62.5 | |||
| GROUP 5 | 9a | 62.5 | 125 | 125 | >250 | >250 | >250 | 250 | 500 | 250 | 500 | 61.5 |
| 62.5 | 125 | 125 | >250 | >250 | >250 | 500 | 500 | 250 | 500 | |||
| 9b | 32 | 125 | 125 | 250 | 250 | 250 | 7.81 | 7.81 | 1.95 | 500 | ND | |
| 62.5 | 125 | 125 | 250 | 250 | 250 | 7.81 | 7.81 | 1.95 | 500 | |||
| 9c | 62.5 | 62.5 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | 23.8 | |
| 62.5 | 125 | 62.5 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| 9d | 62.5 | 62.5 | 62.5 | >125 | >125 | 7.81 | >500 | >500 | >500 | >500 | ND | |
| 62.5 | 62.5 | 62.5 | >125 | >125 | 7.81 | >500 | >500 | >500 | >500 | |||
| 9e | 32 | 32 | 32 | 125 | 125 | 125 | >250 | >250 | >250 | >500 | ND | |
| 32 | 62.5 | 62.5 | 125 | 125 | 125 | >250 | >250 | >250 | >500 | |||
| 9f | 32 | 32 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | 23.5 | |
| 32 | 32 | 32 | >125 | >125 | >125 | >500 | >500 | >500 | >500 | |||
| GROUP 6 | 9g | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 24 |
| 9h | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 14.2 | |
| 9i | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 37.3 | |
| 9j | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 35.7 | |
| INH | 0.5 | >250 | >250 | – | – | – | – | – | – | – | – | |
| 0.5 | >250 | >250 | ||||||||||
| FLU | – | – | – | 0.12 | 3.91 | 1.95 | – | – | – | – | – | |
| >125 | 15.62 | 3.91 | ||||||||||
| PEN | – | – | – | – | – | – | 0.24 | 125 | 31.62 | 7.81 | – | |
| 0.24 | 125 | 125 | 15.62 | |||||||||
| CPF | – | – | – | – | – | – | 0.98 | 500 | 250 | 0.98 | – | |
| 0.98 | 500 | 250 | 0.98 | |||||||||
| DCMU | – | – | – | – | – | – | – | – | 1.9 | |||
The MIC determinations were performed according to the CLSI reference protocol: amycobacteria (IC90 value), bM27-A2 for yeasts (IC80 value), cM38-A for moulds-fungi (IC50 value) and dbacteria (IC90 value), ecompounds precipitated during the experiments.
Compounds 8h and 8k/l (Group 2) exhibited the highest activity against M. tuberculosis. Based on these observations, it can be concluded that chlorination in the C(4) position results in less active compounds, substitution in the position C'(4) of the anilide part is essential for high activity, and 4-Cl (Group 2) is more advantageous than 4-Br (Group 4). In general, compounds within Group 1 (3-Cl substituted) showed negligible activity. The influence of chlorine in the position C'(4) of the anilide part seems to be dominant in Group 3 (series 5-Cl/3,4-Cl), see activity in Table 2. Lipophilicity seems to be only a secondary parameter; in the same way, the use of electronic parameter σ of R2 substituent does not improve the results of SAR discussion. High activity of 8h cannot be explained on the basis of simple pattern.
Compounds 8k/l and 8g (Group 2 and Group 1) and 9e/f (Group 5) showed the highest activity against M. avium and other compounds 8i/j, 8a, 8s and 9d demonstrated medium activity. Based on these facts, it can be assumed that the substitution of R3, especially by benzyl moiety, as well as preferential substitution by chlorine, especially in C'(4) position of the anilide part of a molecule, is important for high activity. Chlorination of C(4) or C(5) position is only a secondary parameter. Substitution in C'(4) by bromine (Group 4) or disubstitution in C'(3,4) (Group 3) by chlorine causes activity decrease compared with Group 2. Lipophilicity seems to be also one of the parameters affecting antimycobacterial activity. The most active compounds from all groups show log k about 0.70. Stereoisomerism considerably influences antimycobacterial activity against M. avium as discussed below.
Compounds 8l, 8v and 9f (Group 2, Group 4 and Group 5) showed the highest antimycobacterial activity against M. kansasii, and other compounds, 8k, 9e, 8i/j, 8g and 8c, expressed substantial activity as well. Based on these facts, it can be concluded that chlorination of C(4) or C(5) position is only a secondary parameter, while substitution of C'(4) position is more advantageous for high activity, and substitution by chlorine is preferred. Disubstitution of C'(3,4) position is connected with activity decrease. The substitution of R3, particularly by benzyl or isopropyl moiety, is associated with higher activity, which can be positively influenced by lipophilicity increase. Similarly, based on Table 1 and Table 2 it can be considered that antimycobacterial activity against M. kansasii is also partly affected by bulk parameters expressed as MR (reflecting bulkiness) of R3 substituents [33]. It seems that antimycobacterial activity is increased by the increase of the bulkiness of individual R3 substituents in the molecule.
From the set of 28 tested compounds the dependence of activity (log 1/MIC, 14 h experiment) on log k related to antimycobacterial activity against M. kansasii (see Figure 2) was linear for 24 compounds (compounds 8b/c and 8q/r were excluded from the correlation). Introduction of σ parameter did not improve the results of statistical analysis:
log(1/MIC) = 3.186(±0.091) + 1.574(±0.136) log k
r = 0.926, s = 0.092, F = 132.07, n = 24
log (1/MIC) = 3.232(±0.095) +1.594(±0.135) log k – 0.185(±0.135) σ
r = 0.933, s = 0.090, F = 70.12, n = 24

Figure 2.
Dependence of antimycobacterial activity against M. kansasii (log 1/MIC [mol/L], 14 h experiment) on compounds lipophilicity expressed as log k.
A very important parameter influencing activity is stereoisomerism, because individual enantiomers demonstrate considerable difference in their antituberculotic/antimycobacterial activity, e.g. enantiomers 8b/c, 8f/g, 8k/l, 8o/p, 8u/v, 9c/d and 9e/f showed different antimycobacterial activity. These differences are especially evident for antimycobacterial activity against the atypical strains M. avium and M. kansasii. More often (R)-enantiomers showed higher activity. The determined differences in antimycobacterial activity for individual R/S-enantiomers cannot be explained on the basis of the results presented here. Due to these facts it can be assumed that stereospecific bond is formed between the compound and an enzyme in Mycobacterium sp. with subsequent enzyme inhibition.
2.3.2. In vitro antifungal susceptibility testing
The evaluation of in vitro antifungal activity of the synthesized compounds was performed against eight fungal strains, but only activity against Candida tropicalis 156 (CT), C. krusei ATCC 6258 (CK) and against Trichophyton mentagrophytes 445 (TM) is shown in Table 2. Generally, all the compounds expressed only moderate antifungal activity, which was caused by low solubility of compounds in the testing medium and their precipitation during the incubation period, therefore no definite structure-activity relationships could be established. Only four compounds from Group 1, 8f/g, 8e and 8b/c, showed very high antifungal activity comparable with or higher than the standard fluconazole (FLU) against C. krusei and against T. mentagrophytes 445. More often (R)-enantiomers showed higher activity, e.g. 8c/b, 8e/d, 9d/c, nevertheless, the determined differences in antibacterial activity for individual R/S-enantiomers cannot be explained on the basis of the results presented here.
Generally, according to the results from Table 2, it can be concluded that chloration of C(4) position or disubstitution R2 = 3,4-Cl considerably decreases solubility, and therefore it is not advantageous. The substitution R2 = 3-Cl is essential for high activity. The substitution of R3 modulates lipophilicity, and it seems to be an important parameter. Based on Table 2 it can be concluded that a lipophilicity decrease results in a decrease of antifungal activity: log k = 0.70 (8f/g) > 0.61 (8e) > 0.41 (8b/c).
When the dependence of activity (log 1/MIC, 24 h experiment) on log k related to antifungal activity of tested compounds against C. krusei (which is the most inhibited fungi strain) was studied, the dependence was linear only for Group 1 (3-Cl substituted derivatives):
log (1/MIC) = 2.901(±0.085) + 3.986(±0.149) log k
r = 0.998, s = 0.061, F = 711.38, n = 5
The above discussed antimycobacterial activity against M. kansasii for the whole series of the tested compounds is mainly dependent on lipophilicity. In contrast to the antimycobacterial activity, the effect of σ parameter of R2 substituent on the activity of evaluated compounds against C. krusei was much more expressive than that of log k:
log (1/MIC) = 1.732(±0.780) + 8.863(±2.575) σ
r = 0.754, s = 0.614, F = 11.84, n = 11
log (1/MIC) = 4.276 (±1.070) + 0.102 (±1.683) log k
r = 0.203, s = 0.934, F = 0.004, n = 11
Multilinear correlation was found to fit the biological activity of compounds as a function of compound lipophilicity log k and σ constant of R2 substituent, and the results of statistical analysis showed that the activity was affected by both parameters.
log (1/MIC) = -0.038 (±1.185) + 1.986 (±1.077) log k + 10.743(±2.507) σ
r = 0.834, s = 0.546, F = 9.19, n = 11
2.3.3. In vitro antibacterial susceptibility testing
Twenty-eight benzamides were tested for their in vitro antibacterial activity against four Gram positive bacterial strains Staphylococcus aureus CCM 4516/08 (SA), methicilin resistant S. aureus H 5996/08 (MRSA), S. epidermidis H 6966/08 (SE) and Enterococcus sp. J 14365/08 (EF). The results are shown in Table 2. Generally, all the compounds expressed only moderate antibacterial activity caused by the low solubility of compounds in the testing medium and their precipitation during the incubation period, as it was mentioned above. In summary, five compounds 8v, 8j, 8e and 9b showed notable antibacterial activity comparable with or higher than the both standards penicillin G and ciprofloxacin against S. aureus methicilin resistant and S. epidermidis.
Based on the results from Table 2, especially halogenation of C'(4) position (R2 = 4-Br or 4-Cl) seem to be important for antibacterial activity. Chloration of C(4) position decreased solubility compared with chloration of C(5) position. Stereoisomerism is probably also an important parameter influencing the activity, because individual enantiomers demonstrate considerable difference in their antibacterial activity, e.g., enantiomers 8u/v, 8d/e, 8i/j and 9a/b. More often (R)-enantiomers showed higher activity (the same as M. kansasii). R3 substitution modulates physico-chemical properties of the discussed compounds. According to Table 2, a bulkier substituent is probably more advantageous. A more detailed SAR study cannot be established due to the small number of effective compounds.
2.3.4. Inhibition of photosynthetic electron transport (PET) in spinach chloroplasts
Seventeen compounds were tested for their PET in spinach (Spinacia oleracea L.) chloroplasts, and they showed some wide-range activity, see Table 2. Compounds 8f (IC50 = 12.3 µmol/L), 8e (IC50 = 13.8 µmol/L) and 9h (IC50 = 14.2 µmol/L) were the most effective inhibitors of the tested compounds, which PET-inhibiting activity in spinach chloroplasts was measured. The activity of the rest of the studied compounds was moderate or low relative to the standard.
Due to the fact that PET-inhibiting data are available only from Group 1 and Groups 5 + 6, SAR study involves the first of these series, but a general SAR hypothesis may be proposed. Generally, it can be concluded that C(4) substitution is more advantageous then C(5) substitution. In the C(5) series C´(3) substitution is important for PET activity. Compounds within the C(5) series with substitution in C´(4) by Cl or Br exhibit similar activity, which is lower than that of C´(3) substituted compounds, but solubility in the testing medium is drastically reduced in the case of C´(4) substitution. Disubstitution in C´(3,4) by Cl also causes lower solubility. Bulkiness and branching of R3 substitution are also important. It can be noticed that mainly (S)-enantiomers are active in PET assay (compare 8d/e or 8k/l) and only 8e, 9f, 9g are slightly active (R)-enantiomers. The correlations between PET-inhibiting activity and lipophilicity could be expressed by equation:
log (1/IC50) = 1.925(±0.427) + 8.302(±1.497) log k – 6.270(±1.266) (log k)2
r = 0.856, s = 0.191, F = 19.17, n =17
For PET inhibition of tested compounds the lipophilicity was determinant because the introduction of σ parameter did not improve the results of statistical analysis:
log (1/IC50) = 1.903(±0.460) + 8.365(±1.595) log k – 6.345(±1.383) (log k)2 + 0.0476(±0.279) σ
r = 0.856, s = 0.198, F = 11.91, n = 17
3. Experimental
3.1. Synthesis
3.2. Lipophilicity determination by HPLC (capacity factor k/calculated log k)
A Waters Alliance 2695 XE HPLC separation module and a Waters Photodiode Array Detector 2996 (Waters Corp., Milford, MA, USA) were used. A Symmetry® C18 5 μm, 4.6 × 250 mm, Part No. WAT054275 (Waters Corp., Milford, MA, USA) chromatographic column was used. The HPLC separation process was monitored by Empower™ 2 Chromatography Data Software, Waters 2009 (Waters Corp., Milford, MA, USA). A mixture of MeOH p.a. (70%) and H2O-HPLC – Mili-Q Grade (30%) was used as a mobile phase. The total flow of the column was 1.0 mL/min, injection volume, 30 μL, column temperature, 30 °C and sample temperature, 10 °C. The detection wavelength of 210 nm was chosen. The KI methanolic solution was used for the dead time (tD) determination. Retention times (tR) were measured in minutes. The capacity factors k were calculated using the Empower™ 2 Chromatography Data Software according to formula k = (tR - tD)/tD, where tR is the retention time of the solute, whereas tD denotes the dead time obtained using an unretained analyte. Log k, calculated from the capacity factor k, is used as the lipophilicity index converted to log P scale. The log k values of the individual compounds are shown in Table 1.
3.3. Lipophilicity calculations
Log P, i.e. the logarithm of the partition coefficient for n-octanol/water, was calculated using the programs CS ChemOffice Ultra ver. 10.0 (CambridgeSoft, Cambridge, MA, USA) and ACD/LogP ver. 1.0 (Advanced Chemistry Development Inc., Toronto, Canada). Clog P values (the logarithm of n-octanol/water partition coefficient based on established chemical interactions) were generated by means of CS ChemOffice Ultra ver. 10.0 (CambridgeSoft, Cambridge, MA, USA) software. The results are shown in Table 1.
3.4. In vitro antimycobacterial evaluation
The in vitro antimycobacterial activity of all prepared compounds was evaluated against Mycobacterium tuberculosis MTB CNCTC My 331/88 (identical with H37RV and ATCC 27294, dilution of the strain was 10−3 μmol/L), Mycobacterium avium MA CNCTC My 330/88 (identical with ATCC 25291, dilution of the strain was 10−5 μmol/L) and Mycobacteriumkansasii MK CNCTC My 235/80 (identical with ATCC 12478, dilution of the strain was 10−4 μmol/L) in the Laboratory for mycobacterial diagnostics and TB, Institute of Public Health in Ostrava, Czech Republic. All strains were obtained from the Czech National Collection of Type Cultures (CNCTC). Antimycobacterial activities were determined in the Sula semisynthetic medium (Sevac, Prague, Czech Republic). Each strain was simultaneously inoculated into a Petri plates containing the Lowenstein-Jensen medium for the control of sterility of the inoculum and its growth. The tested compounds were added to the medium as DMSO solutions. The following concentrations were used: 250, 125, 62, 32, 16, 8, 4, 2, and 1 μmol/L. Inoculated plates kept in microtone bags were incubated at 37 °C. Reading was carried out on a stand with a bottom magnifying mirror, macroscopically, with the use of magnifying glass. The growth in plates was evaluated after 14 and 21 days of the incubation. The growth of the colonies in a control well plate, corresponding to the growth into 100 colonies in the control Lowenstein-Jensen medium was considered as an optimum dilution for the evaluation of the results. In the course of the test evaluation, the MIC (μmol/L) definition was considered as the lowest substance concentration at which the inhibition of growth of mycobacteria occurred. [34] Isoniazid (INH) was used as the standard as it is a clinically used anti-mycobacterial drug. The results are shown in Table 2. The MIC for mycobacteria was defined as a 90% or greater (IC90) reduction of growth in comparison with the control. The MIC/IC90 value is routinely and widely used in bacterial assays and is a standard detection limit according to the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS, www.clsi.org/).
3.5. In vitro antifungal susceptibility testing
The broth microdilution test [35,36,37] was used for the assessment of in vitro antifungal activity of the synthesized compounds against Candida albicans ATCC 44859, C. tropicalis 156, C. krusei E28, C. glabrata 20/I, Trichosporon asahii 1188, Trichophyton mentagrophytes 445, Aspergillus fumigantus 231 and Absidia corymbifera 272. Fluconazole (FLU) was used as a standard, since it is a clinically used antimycotic drug. The procedure was performed with a two-fold dilution of the compounds in RPMI 1640 (Sevapharma a.s., Prague, Czech Republic) buffered to pH 7.0 with 0.165 mol of 3-morpholino-propane-1-sulfonic acid (MOPS, Sigma, Germany). The final concentrations of the compounds ranged from 500 to 0.975 μmol. Drug–free controls were included. The MIC determination was performed according to the CLSI reference protocol M27-A2 for yeasts (IC80 value) and M38-A for moulds (IC50 value). IC50 values were defined as a 50% reduction of growth in comparison with the control, and IC80 values were defined as a 80% reduction of growth in comparison with the control. The trays were incubated at 35 °C, and MICs were read visually for filamentous fungi and photometrically for yeasts as absorbance at 540 nm after 24 h and 48 h. The MIC values for the dermatophytic strain (T. mentagrophytes) were determined after 72 h and 120 h. MICs were determined twice and in duplicate. The results are summarized in Table 2.
3.6. In vitro antibacterial susceptibility testing
The synthesized compounds were evaluated for in vitro antibacterial activity against Staphylococcus aureus CCM 4516/08, methicilin resistant Staphylococcus aureus H 5996/08, Staphylococcus epidermidis H 6966/08 and Enterococcus sp. J 14365/08. Penicillin G (PEN) and ciprofloxacin (CPF) were used as standards since they are clinically used antibacterial drugs. All strains were sub-cultured on nutrient agar (HiMedia) and maintained on the same medium at 4 °C. Prior to testing, each strain was passaged onto nutrient agar and bacterial inocula were prepared by suspending a small portion of bacterial colony in sterile 0.85% saline. The cell density was adjusted to 0.5 McFarland units using a densitometer (Densi-La-Meter, PLIVA Lachema Diagnostika, Czech Republic). The final inoculum was made by 1:20 dilution of the suspension with the test medium (Mueller-Hinton broth). The compounds were dissolved in DMSO and the anti-bacterial activity was determined using Mueller-Hinton broth (MH broth, HiMedia, pH 7.0 ± 0.2). Controls consisted of MH broth and DMSO alone. The final concentration of DMSO in the MH broth did not exceed 1% (v/v) of the total solution composition. The activity of the studied compounds was determined as the minimal inhibition concentration (MIC) according to NCCLS guidelines [38] using broth microdilution test. The MICs were defined as 90% inhibition of bacterial growth compared to the control and were determined after 24 and 48 h of static incubation at 37 °C. After incubation MICs were read visually as an absorbance at 540 nm. The results are shown in Table 2.
3.7. Study of inhibition photosynthetic electron transport (PET) in spinach chloroplasts
Chloroplasts were prepared from spinach (Spinacia oleracea L.) according to Masarovicova and Kralova [39]. The inhibition of photosynthetic electron transport (PET) in spinach chloroplasts was determined spectrophotometrically (Genesys 6, Thermo Scientific, USA), using an artificial electron acceptor 2,6-dichlorophenol-indophenol (DCIPP) according to Kralova et al. [40], and the rate of photosynthetic electron transport was monitored as a photoreduction of DCPIP. The measurements were carried out in phosphate buffer (0.02 mol/L, pH 7.2) containing sucrose (0.4 mol/L), MgCl2 (0.005 mol/L) and NaCl (0.015 mol/L). The chlorophyll content was 30 mg/L in these experiments and the samples were irradiated (~100 W/m2) from 10 cm distance with a halogen lamp (250 W) using a 4 cm water filter to prevent warming of the samples. The studied compounds were dissolved in DMSO due to their limited water solubility. The applied DMSO concentration (up to 4%) did not affect the photochemical activity in spinach chloroplasts. The inhibitory efficiency of the studied compounds was expressed by IC50 values, i.e. by molar concentration of the compounds causing 50% decrease in the oxygen evolution rate relative to the untreated control. The comparable IC50 value for a selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, DCMU (Diurone®) was about 1.9 μmol/L [41]. The results are summarized in Table 2.
4. Conclusions
A series of twenty-two 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides and ten 4-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides is described. Their lipophilicity was determined using a well established RP-HPLC method. The prepared compounds were tested for their antimycobacterial, antifungal and antibacterial activity as well as for their ability to inhibit photosynthetic electron transport (PET) in spinach chloroplasts (Spinacia oleracea L.). For all the compounds the relationships between their lipophilicity and chemical structure as well as structure-activity relationships were discussed. N-{(1R)-1-benzyl-2-[(4-chlorophenyl)amino]-2-oxo-ethyl}-5-chloro-2-hydroxybenzamide (8l, 32 μmol/L) and N-{(1R)-1-benzyl-2-[(4-chlorophenyl)-amino]-2-oxoethyl}-4-chloro-2-hydroxybenzamide (9f, 32 μmol/L) showed the highest antitubercular activity and higher antimycobacterial effect than the standard isoniazid. N-{(1S/R)-1-benzyl-2-[(3-chlorophenyl)amino]-2-oxoethyl}-5-chloro-2-hydroxybenzamide (8f, 8g, 3.9 μmol/L) and 5-chloro-N-[(1R)-1-{[(3-chlorophenyl)amino]-carbonyl}-2-methylpropyl)]-2-hydroxybenzamide (8e, 7.81 μmol/L) demonstrated higher antifungal activity against Candida krusei and 5-chloro-N-{(1R)-2-[(3-chlorophenyl)amino]-1-methyl-2-oxoethyl}-2-hydroxybenzamide (8c, 0.49 μmol/L) showed higher antifungal activity against Trichophyton mentagrophytes than the standard fluconazole. 5-Chloro-N-[(1S/R)-1-{[(4-chlorophenyl)amino]carbonyl}-2-methylpropyl)]-2-hydroxybenzamide (compound 8j, 7.81 μmol/L) exhibited higher activity against methicillin-resistant Staphylococcus aureus than penicillin G and ciprofloxacin. 4-Chloro-N-{(1R)-2-[(4-chlorophenyl)-amino]-1-methyl-2-oxoethyl}-2-hydroxy benzamide (9b, 7.81 μmol/L and 1.95 μmol/L) showed higher activity against methicillin-resistant Staphylococcus aureus and S. epidermidis than penicillin G and ciprofloxacin. N-{(1S)-1-benzyl-2-[(3-chlorophenyl)amino]-2-oxoethyl}-5-chloro-2-hydroxybenz-amide (8f, 12.3 μmol/L) and 5-chloro-N-[(1S)-1-{[(3-chlorophenyl)amino]-carbonyl}-2-methylpropyl)]-2-hydroxybenzamide (8d, 13.8 μmol/L) showed the highest PET-inhibition activity among the discussed compounds. It can be concluded that (R)-enantiomers demonstrated higher antimicrobial activity compared with (S)-enantiomers, while (S)-enantiomers showed higher inhibition of photosynthetic electron transport in spinach chloroplasts.
Acknowledgements
This study was supported by the IGA NS 10367-3, the Ministry of Education of the Czech Republic (MSM 0021627501 and MSM 0021620822) and by Sanofi-Aventis Pharma Slovakia.
References
- Vinsova, J.; Imramovsky, A. Salicylanilides: Still a topical potential antibacterially active group. Ces. Slov. Farm. 2004, 53, 294–299. [Google Scholar]
- Dahlgren, M.K.; Kauppi, A.M.; Olsson, I.M.; Linusson, A.; Elofsson, M. Design, synthesis, and multivariate quantitative structure–activity relationship of salicylanilidess–potent inhibitors of type III secretion in Yersinia. J. Med. Chem. 2007, 50, 6177–6188. [Google Scholar] [CrossRef]
- Stephenson, K.; Yamaguchi, Y.; Hoch, J.A. The mechanism of action of inhibitors of bacterial two-component signal transduction systems. J. Biol. Chem. 2000, 275, 38900–38904. [Google Scholar] [CrossRef]
- Vinsova, J.; Imramovsky, A.; Buchta, V.; Ceckova, M.; Dolezal, M.; Staud, F.; Jampilek, J.; Kaustova, J. Salicylanilide acetates: Synthesis and antibacterial evaluation. Molecules 2007, 12, 1–12. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Dolezal, R.; Jampilek, J.; Kaustova, J.; Kunc, F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg. Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef]
- Ferriz, J.M.; Vavrova, K.; Kunc, F.; Imramovsky, A.; Stolarikova, J.; Vavrikova, E.; Vinsova, J. Salicylanilide carbamates: Antitubercular agents active against multidrug-resistant Mycobacterium tuberculosis strains. Bioorg. Med. Chem. 2010, 18, 1054–1061. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Buchta, V.; Jampilek, J. Salicylanilide esters of N-protected amino acids as novel antimicrobial agents. Bioorg. Med. Chem. Lett. 2009, 19, 348–351. [Google Scholar] [CrossRef]
- Brown, M.E.; Fitzner, J.N.; Stevens, T.; Chin, W.; Wright, C.D.; Boyce, J.P. Salicylanilides: Selective inhibitors of interleukin-12p40 production. Bioorg. Med. Chem. 2008, 16, 8760–8764. [Google Scholar] [CrossRef]
- Liechti, C.; Sequin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef]
- Deng, W.; Guo, Z.; Guo, Y.; Feng, Z.; Jiang, Y.; Chu, F. Acryolylamino-salicylanilides as EGFR PTK inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 469–472. [Google Scholar] [CrossRef]
- Kamath, S.; Buolamwini, J.K. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Med. Res. Rev. 2006, 26, 569–594. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Kunes, J.; Pour, M.; Dolezal, M. Salicylanilide esterification: Unexpected formation of novel seven-membered rings. Tetrahedron Lett. 2006, 47, 5007–5011. [Google Scholar] [CrossRef]
- Vinsova, J.; Imramovsky, A.; Kratky, M.; Ferriz, J.M.; Palat, K.; Lycka, A.; Ruzicka, A. An unprecedented rearrangement of salicylanilide derivatives: Imidazolinone intermediate formation. Tetrahedron Lett. 2010, 51, 23–26. [Google Scholar]
- Imramovsky, A.; Ferriz, J.M.; Pauk, K.; Kratky, M.; Vinsova, J. Synthetic route for the preparation of 2-hydroxy-N-[1-(2-hydroxyphenylamino)-1-oxoalkan-2-yl]benzamides. J. Comb. Chem. 2010, 12, 414–416. [Google Scholar] [CrossRef]
- Good, N.E. Inhibitors of the Hill reaction. Plant Physiol. 1961, 36, 788–803. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Cizmarik, J. Inhibitory effect of piperidinoethylesters of alkoxyphenylcarbamic acids on photosynthesis. Gen. Physiol. Biophys. 1992, 11, 261–267. [Google Scholar]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibitory effects of substituted benzanilides on photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 1999, 53, 328–331. [Google Scholar]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibition of photosynthetic electron transport in spinach chloroplasts by 3- and 4-halogeno substituted benzanilides and thiobenzanilides. J. Trace Microprobe Technol. 2000, 18, 251–256. [Google Scholar]
- Black, C.C. Photosynthetic phosphorylation and associated reactions in the presence of a new group of uncouplers: Salicylanilides. Biochim. Biophys. Acta 1968, 162, 294–296. [Google Scholar] [CrossRef]
- Dolezal, M.; Miletin, M.; Kunes, J.; Kralova, K. Substituted amides of pyrazine-2-carboxylic acids, their synthesis and biological activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Dolezal, M.; Palek, L.; Vinsova, J.; Buchta, V.; Jampilek, J.; Kralova, K. Substituted pyrazinecarboxamides: Synthesis and biological evaluation. Molecules 2006, 11, 242–256. [Google Scholar] [CrossRef]
- Dolezal, M.; Tumova, L.; Kesetovicova, D.; Tuma, J.; Kralova, K. Substituted N-phenylpyrazine-2-carboxamides, their synthesis and evaluation as herbicides and abiotic elicitors. Molecules 2007, 12, 2589–2598. [Google Scholar] [CrossRef]
- Dolezal, M.; Cmedlova, P.; Palek, L.; Vinsova, J.; Kunes, J.; Buchta, V.; Jampilek, J.; Kralova, K. Synthesis and antimycobacterial evaluation of substituted pyrazinecarboxamides. Eur. J. Med. Chem. 2008, 43, 1105–1113. [Google Scholar] [CrossRef]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; Csollei, J.; Richardson, D.R.; Jampilek, J. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J.; Osicka, Z.; Kunes, J.; Vejsova, M.; Buchta, V.; Dohnal, J.; Jampilek, J.; Kralova, K. Synthesis, antimycobacterial, antifungal and photosynthesis-inhibiting activity of chlorinated N-phenylpyrazine-2-carboxamides. Molecules 2010, 15, 8567–8581. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Miletin, M.; Dolezal, M. Inhibition of photosynthetic electron transport in spinach chloroplasts by 2,6-disubstituted pyridine-4-thiocarboxamides. Chem. Pap. 2002, 56, 214–217. [Google Scholar]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Govindjee, S. Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Kerns, E.H.; Li, D. Drug-like Properties: Concept, Structure Design and Methods; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Norrington, F.E.; Hyde, R.M.; Williams, S.G.; Wotton, R. Physicochemical-activity relations in practice. 1. Rational and self-consistent data bank. J. Med. Chem. 1975, 18, 604–607. [Google Scholar] [CrossRef]
- Kaustova, J. Quantitative micromethod for drug susceptibility testing of mycobacteria in Sula’s medium. Klin. Mikrobiol. Infeck. Lek. 1997, 3, 115–117. [Google Scholar]
- Sheehan, D.J.; Espinel-Ingroff, A.; Steele, M.; Webb, C.D. Antifungal susceptibility testing of yeasts: A brief overview. Clin. Infect. Dis. 1993, 17, 494–500. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Testing of Yeasts: Approved Standard M27-A2, 2nd edNational Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002.
- National Committee for Clinical Laboratory Standards. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts: Approved Guideline M44-A; National Committee for Clinical Laboratory Standards, Wayne, PA, USA, 2004.
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically Approved Standard: Approved Standard M7-A4, 4th edNational Committee for Clinical Laboratory Standards: Villanova, PA, USA, 1997.
- Masarovicova, E.; Kralova, K. Approaches to measuring plant photosynthesis activity. In Handbook of Photosynthesis, 2nd; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- Fedke, C. Biochemistry and Physiology of Herbicide Action; Springer Verlag: New York, NY, USA, 1982. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).