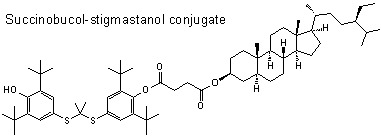
Succinobucol’s New Coat — Conjugation with Steroids to Alter Its Drug Effect and Bioavailability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Crystal Structure Determination


2.3. Toxicity Tests

2.4. Stability Tests of Succinobucol and Conjugates 3–6 under Acidic Conditions
2.5. 1,1-Diphenyl-2-picrylhydrazyl Radical (DPPH) Scavenging Activity and its Mechanism of Action
| Compound | Ascorbic acid | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| EC50 | 0.27 | 0.20 | 0.17 | 0.20 | 0.14 | 0.27 | 0.26 |
| ARP | 3.7 | 5.0 | 5.9 | 4.9 | 7.1 | 3.7 | 3.8 |




3. Experimental
3.1. Chemistry
3.1.1. Preparation of Succinobucol [4-{2,6-di-tert-butyl-4-[(1-{ [3-tert-butyl-4-hydroxy-5-(propan-2-yl)phenyl] sulfanyl}ethyl)sulfanyl]phenoxy}-4-oxobutanoic acid] (Scheme 1)
3.1.2. Preparation of Sterol Conjugates of Succinobucol 3–6
3.2. Toxicity Test
3.3. Antioxidant Activity — DPPH Scavenging Assay [25,39]
4. Conclusions
Acknowledgments
References and Notes
- Adameova, A.; Xu, Y.J.; Duhamel, T.A.; Tappia, P.S.; Shan, L.; Dhalla, N.S. Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr. Pharm. Des. 2009, 15, 3094–3107. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Gylling, H. Plant stanol and sterol esters in prevention of cardiovascular diseases. Ann. Med. 2004, 36, 126–134. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Tiwari, A. Current and emerging paradigms in the therapeutic management of atherosclerosis. Expert Opin. Ther. Tar. 2008, 12, 1523–1546. [Google Scholar] [CrossRef]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R.; Participants, S.W. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar]
- Miettinen, T.A.; Gylling, H. Plant stanol and sterol esters in prevention of cardiovascular diseases: A review. Int. J. Clin. Pharm. Th. 2006, 44, 247–250. [Google Scholar]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agr. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Ling, W.H.; Jones, P.J.H. Dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Malini, T.; Vanithakumari, G. Rat toxicity studies with β-sitosterol. J. Ethnopharmacol. 1990, 28, 221–234. [Google Scholar] [CrossRef]
- Nissinen, M.; Gylling, H.; Vuoristo, M.; Miettinen, T.A. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am. J. Physiol. Gastr. L. 2002, 282, G1009–G1015. [Google Scholar]
- Sudhop, T.; Sahin, Y.; Lindenthal, B.; Hahn, C.; Luers, C.; Berthold, H.K.; von Bergmann, K. Comparison of the hepatic clearances of campesterol, sitosterol, and cholesterol in healthy subjects suggests that efflux transporters controlling intestinal sterol absorption also regulate biliary secretion. Gut 2002, 51, 860–863. [Google Scholar] [CrossRef]
- von Bergmann, K.; Sudhop, T.; Lütjohann, D. Cholesterol and plant sterol absorption: Recent insights. Am. J. Cardiol. 2005, 96, 10D–14D. [Google Scholar]
- Stocker, R. Molecular mechanisms underlying the antiatherosclerotic and antidiabetic effects of probucol, succinobucol, and other probucol analogues. Curr. Opin. Lipidol. 2009, 20, 227–235. [Google Scholar] [CrossRef]
- Yamashita, S.; Matsuzawa, Y. Where are we with probucol: A new life for an old drug? Atherosclerosis 2009, 207, 16–23. [Google Scholar] [CrossRef]
- Muldrew, K.M.; Franks, A.M. Succinobucol: Review of the metabolic, antiplatelet and cardiovascular effects. Expert Opin. Inv. Drug 2009, 18, 531–539. [Google Scholar] [CrossRef]
- Crim, W.S.; Wu, R.P.; Carter, J.D.; Cole, B.K.; Trace, A.P.; Mirmira, R.G.; Kunsch, C.; Nadler, J.L.; Nunemaker, C.S. Agi-1067, a novel antioxidant and anti-inflammatory agent, enhances insulin release and protects mouse islets. Mol. Cell. Endocrinol. 2010, 323, 246–255. [Google Scholar] [CrossRef]
- Simons, L.A. Additive effect of plant sterol-ester margarine and cerivastatin in lowering low-density lipoprotein cholesterol in primary hypercholesterolemia. Am. J. Cardiol. 2002, 90, 737–740. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Konovalova, G.G.; Tutunov, V.S.; Medvedeva, N.V.; Kotkina, T.I.; Kukharchuk, V.V.; Belenkov, Y.N. Intensification of free radical oxidation of low-density lipoproteins in the plasma of patients with ischemic heart disease receiving β-hydroxy-β-methylglutaryl-coenzyme a reductase inhibitor cerivastatin and inhibition of low-density lipoprotein peroxidation with antioxidant probucol. B. Exp. Biol. Med. 2002, 134, 39–42. [Google Scholar] [CrossRef]
- Salunke, D.B.; Hazra, B.G.; Pore, V.S. Steroidal conjugates and their pharmacological applications. Curr. Med. Chem. 2006, 13, 813–847. [Google Scholar] [CrossRef]
- Bilenko, M.V.; Khil'chenko, A.V.; Konovalova, G.G.; Lankin, V.Z. Effect of antioxidant probucol on cell-mediated LDL oxidation in vitro and in vivo. B. Exp. Biol. Med. 2003, 136, 126–128. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Liu, J.K.; Edamatsu, R.; Ohba, S.; Kadowaki, D.; Mori, A. Probucol scavenged 1,1-diphenyl-2-picrylhydrazyl radicals and inhibited formation of thiobarbituric acid reactive substances. Free Radic. Biol. Med. 1994, 16, 201–206. [Google Scholar] [CrossRef]
- Tikhaze, A.K.; Lankin, B.Z.; Konovalova, G.G.; Shumaev, K.B.; Kaminnyi, A.I.; Kozachenka, A.I.; Gurevich, S.M.; Nagler, L.G.; Zaitseva, T.M.; Kukharchuk, V.V. Antioxidant probucol as an effective scavenger of lipid radicals in low density lipoproteins in vivo and in vitro. B. Exp. Biol. Med. 1999, 128, 818–821. [Google Scholar] [CrossRef]
- Witting, P.K.; Wu, B.J.; Raftery, M.; Southwell-Keely, P.; Stocker, R. Probucol protects against hypochlorite-induced endothelial dysfunction—Identification of a novel pathway of probucol oxidation to a biologically active intermediate. J. Biol. Chem. 2005, 280, 15612–15618. [Google Scholar] [CrossRef]
- Ikonen, S.; Jurček, O.; Wimmer, Z.; Drašar, P.; Kolehmainen, E. Unpublished results.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol. Leb. 1995, 28, 25–30. [Google Scholar]
- Ito, O. Reactions of aromatic thiyl radicals. In S-centered Radicals; Alfassi, Z.B., Ed.; Wiley: New York, NY, USA, 1999; pp. 193–224. [Google Scholar]
- Wardman, P. Thiyl radicals in biology: Their role as a “molecular switch” central to cellular oxidative stress. In S-centered Radicals; Alfassi, Z.B., Ed.; Wiley: New York, NY, USA, 1999; pp. 289–309. [Google Scholar]
- Forgo, P.; Kover, K.E. Gradient enhanced selective experiments in the 1H NMR chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids 2004, 69, 43–50. [Google Scholar] [CrossRef]
- Kovganko, N.V.; Kashkan, Z.N.; Borisov, E.V.; Batura, E.V. 13C NMR spectra of β-sitosterol derivatives with oxidized rings A and B. Chem. Nat. Compd. 1999, 35, 646–649. [Google Scholar] [CrossRef]
- Rubinstein, I.; Goad, L.J.; Clague, A.D.H.; Mulheirn, L.J. 220 MHz NMR-spectra of phytosterols. Phytochemistry 1976, 15, 195–200. [Google Scholar]
- Otwinowski, Z.; Minor, W. Macromolecular crystallography. Part a. In Methods in Enzymology; Carter, C.W.J., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Blessing, R.H.; Weeks, C.M.; Hauptman, H.A.; Smith, G.D.; Teeter, M.M.; Miller, R. Crambin—A direct solution for a 400-atom structure. Acta Cryst. D 1995, 51, 33–38. [Google Scholar]
- Burla, M.C.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Polidori, G.; Spagna, R. Sir2002: The program. J. Appl. Cryst. 2003, 36, 1103. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Jass, P.A. Process for preparing probucol derivatives. U.S. Patent 6,323,359,27, Nobember 2001. [Google Scholar]
- Jurček, O.; Lahtinen, M.; Horníček, J.; Wimmer, Z.; Drašar, P.; Kolehmainen, E. Unpublished results.
- Neises, B.; Steglich, W. 4-Dialkylaminopyridines as acylation catalysts. Simple method for esterification of carboxylic acids. Angew. Chem. Int. Ed. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Sieuwerts, A.M.; Klijn, J.G.M.; Peters, H.A.; Foekens, J.A. The MTT tetrazolium salt assay scrutinized - how to use this assay reliably to measure metabolic activity of cell cultures in vitro for the assessment of growth characteristics, IC50 values and cell survival. Eur. J. Clin. Chem. Clin. 1995, 33, 813–823. [Google Scholar]
- Deby, C.; Magottea, G. Essential fatty acids and antioxidizing substances in tissues of mouse. Cr. Soc. Biol. 1970, 164, 2675–2679. [Google Scholar]
- Samples Availability: Samples of compounds 2–6 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jurček, O.; Ikonen, S.; Buřičová, L.; Wimmerová, M.; Wimmer, Z.; Drašar, P.; Horníček, J.; Galandáková, A.; Ulrichová, J.; Kolehmainen, E.T. Succinobucol’s New Coat — Conjugation with Steroids to Alter Its Drug Effect and Bioavailability. Molecules 2011, 16, 9404-9420. https://doi.org/10.3390/molecules16119404
Jurček O, Ikonen S, Buřičová L, Wimmerová M, Wimmer Z, Drašar P, Horníček J, Galandáková A, Ulrichová J, Kolehmainen ET. Succinobucol’s New Coat — Conjugation with Steroids to Alter Its Drug Effect and Bioavailability. Molecules. 2011; 16(11):9404-9420. https://doi.org/10.3390/molecules16119404
Chicago/Turabian StyleJurček, Ondřej, Satu Ikonen, Lucie Buřičová, Martina Wimmerová, Zdeněk Wimmer, Pavel Drašar, Jan Horníček, Adéla Galandáková, Jitka Ulrichová, and Erkki T. Kolehmainen. 2011. "Succinobucol’s New Coat — Conjugation with Steroids to Alter Its Drug Effect and Bioavailability" Molecules 16, no. 11: 9404-9420. https://doi.org/10.3390/molecules16119404
APA StyleJurček, O., Ikonen, S., Buřičová, L., Wimmerová, M., Wimmer, Z., Drašar, P., Horníček, J., Galandáková, A., Ulrichová, J., & Kolehmainen, E. T. (2011). Succinobucol’s New Coat — Conjugation with Steroids to Alter Its Drug Effect and Bioavailability. Molecules, 16(11), 9404-9420. https://doi.org/10.3390/molecules16119404