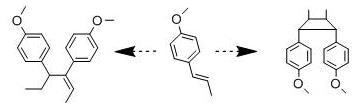
Anethole Isomerization and Dimerization Induced by Acid Sites or UV Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. trans-Anethole treatment with HY zeolites
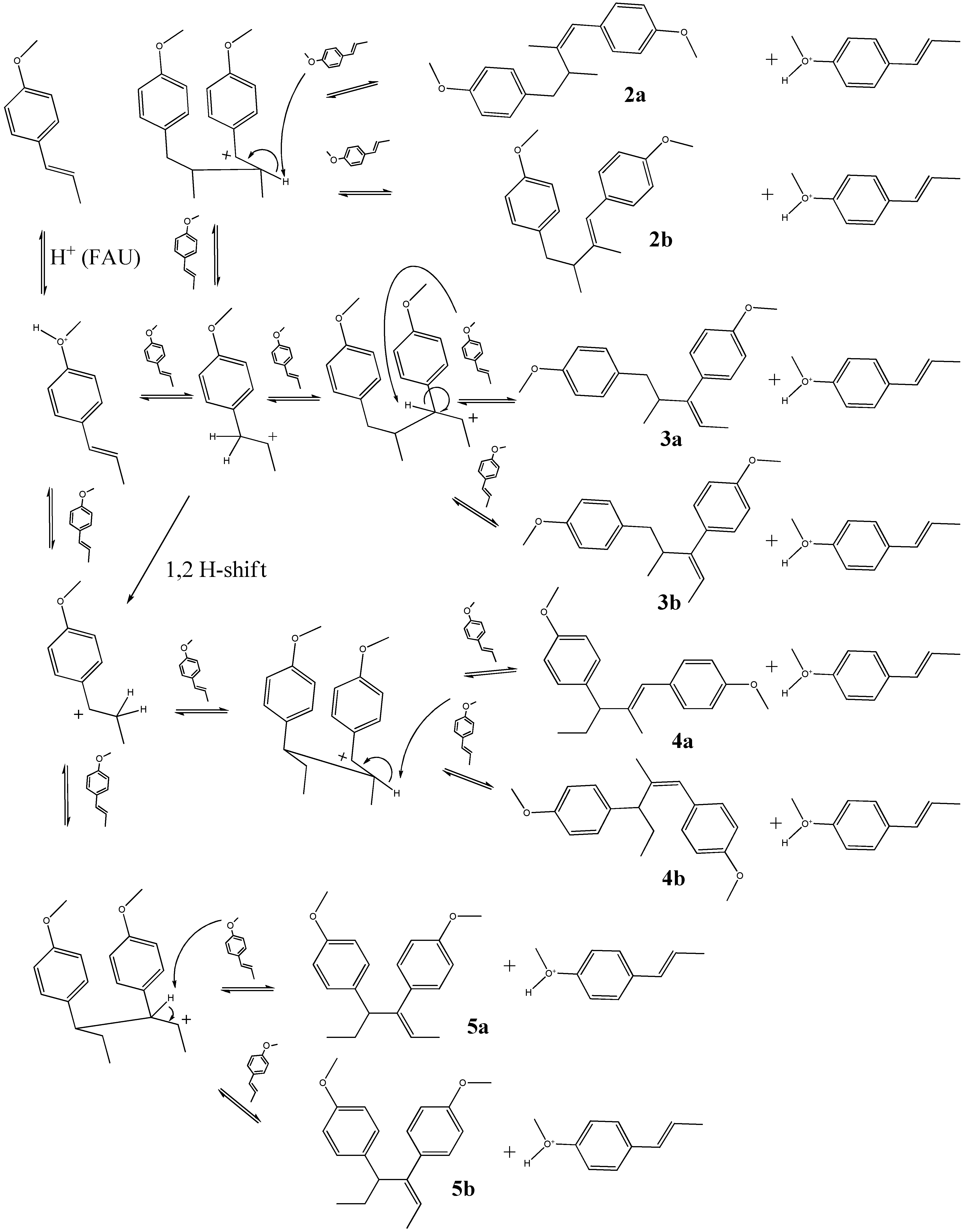
| Relative amount, %a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zeolite | HY1 | HY2 | HY3 | |||||||
| Compd. | T, °C | 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 |
| c is-Anethole | 0.3 ± 0.02 | 1.9 ± 0.24 | 0.8 ± 0.01 | 0.2 ± 0.01 | 1.2 ± 0.80 | 0.9 ± 0.11 | 0.2 ± 0.03 | 1.2 ± 0.08 | 0.4 ± 0.06 | |
| trans-Anethole | 99 ± 0.02 | 45.0 ± 0.80 | 9.0 ± 0.86 | 99 ± 0.01 | 30 ± 23 | 12 ± 1.1 | 99.0 ± 1.7 | 76 ± 1.4 | 8.0 ± 1.9 | |
| 2a | --- | 2 ± 0.17 | 3 ± 0.16 | --- | 2.7 ± 0.92 | 2 ± 0.08 | --- | 0.4 ± 0.18 | 1.4 ± 0.17 | |
| 2b | --- | 1.0 ± 0.08 | 0.9 ± 0.03 | --- | 1.7 ± 0.74 | 4 ± 0.24 | --- | 0.7 ± 0.05 | 2.6 ± 0.27 | |
| 3a.3b | --- | 2.0 ± 0.06 | 9.0 ± 0.03 | --- | 5 ± 2.02 | 9 ± 0.09 | --- | 1.0 ± 0.19 | 23 ± 2.2 | |
| 4a | --- | 1.0 ± 0.08 | 3 ± 0.04 | --- | 1.0 ± 0.38 | 5 ± 0.06 | --- | 0.45 ± 0.05 | 2.2 ± 0.18 | |
| 4b | --- | 2.0 ± 0.05 | 11 ± 0.11 | --- | 3.0 ± 1.14 | 7 ± 1.3 | --- | 0.71 ± 0.06 | 5.9 ± 1.2 | |
| 5a | --- | tr | tr | --- | tr | tr | --- | tr | tr | |
| 5b | --- | 45 ± 0.30 | 63 ± 0.92 | --- | 55 ± 18 | 60 ± 0.4 | --- | 20 ± 1.2 | 57 ± 5.6 | |
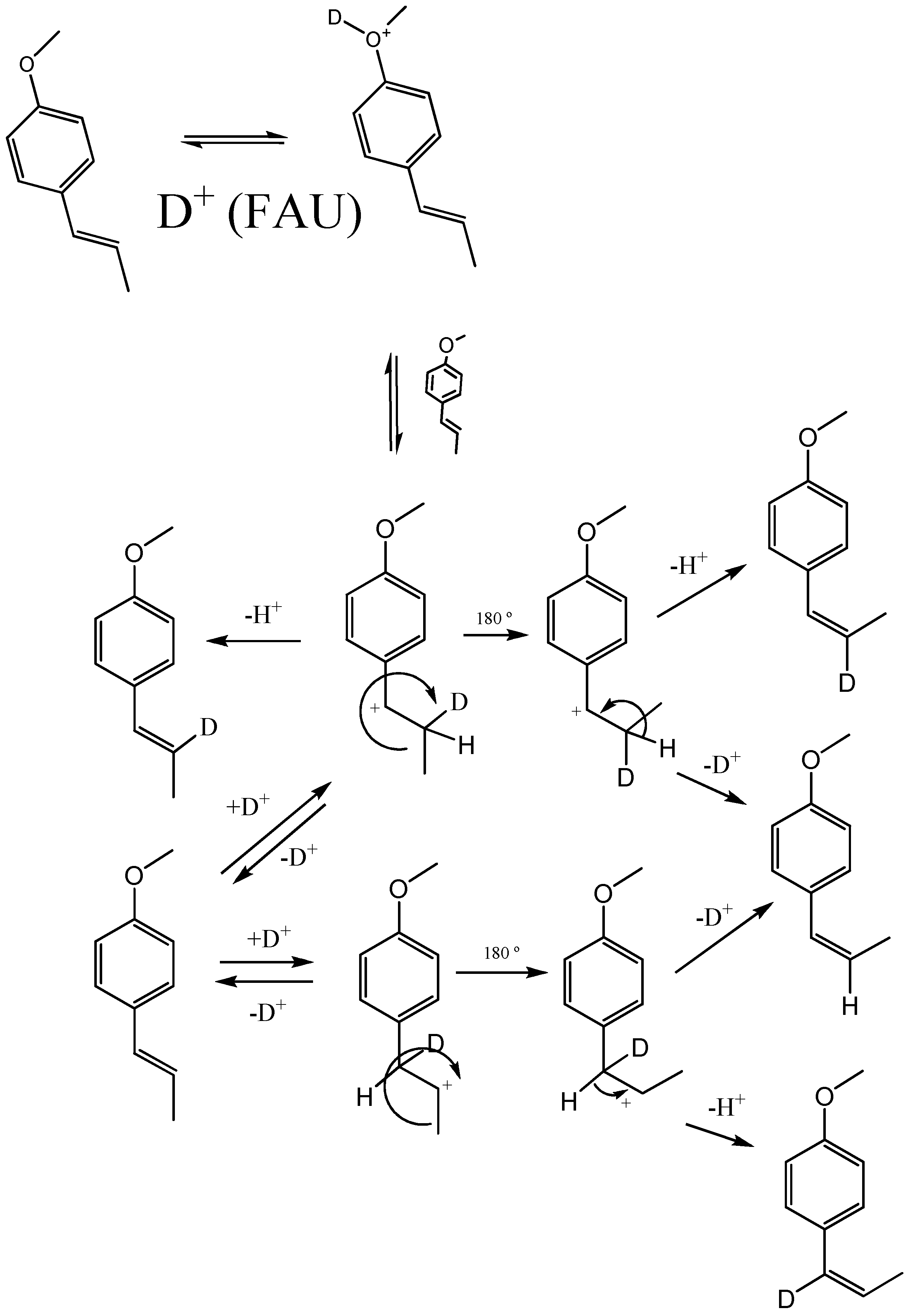
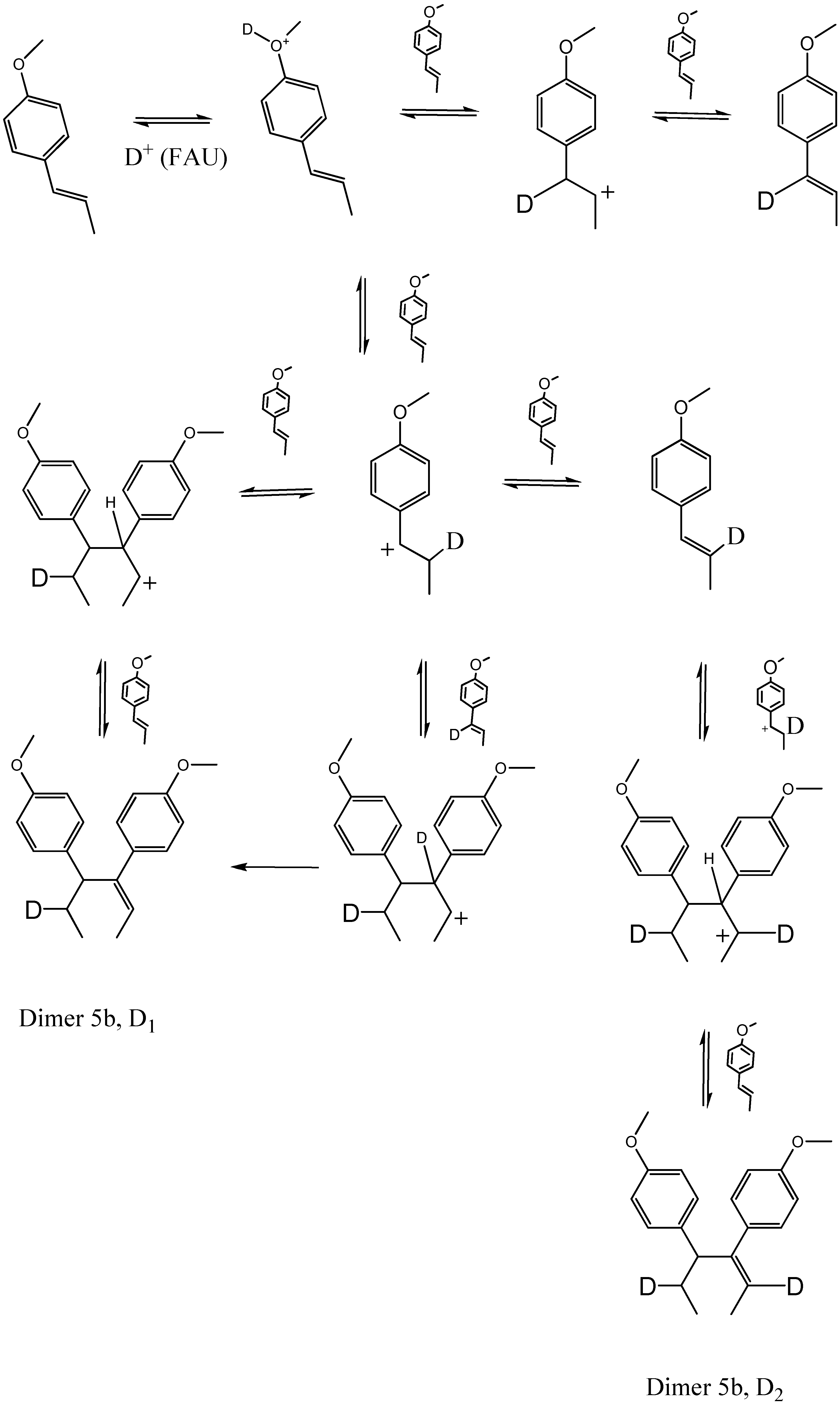
2.2. Transformation of trans-anethole exposed to deuterated zeolite, HDY
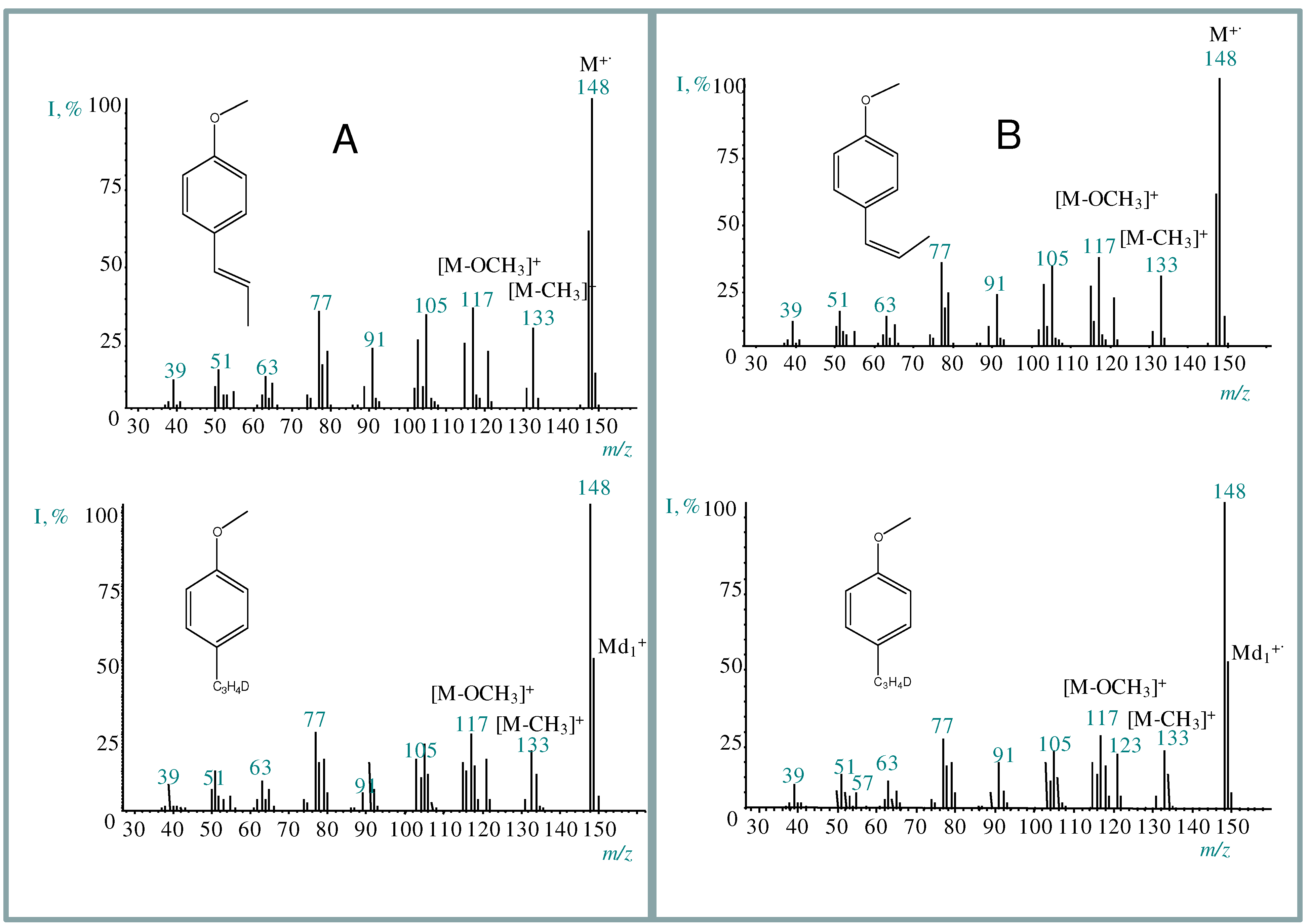
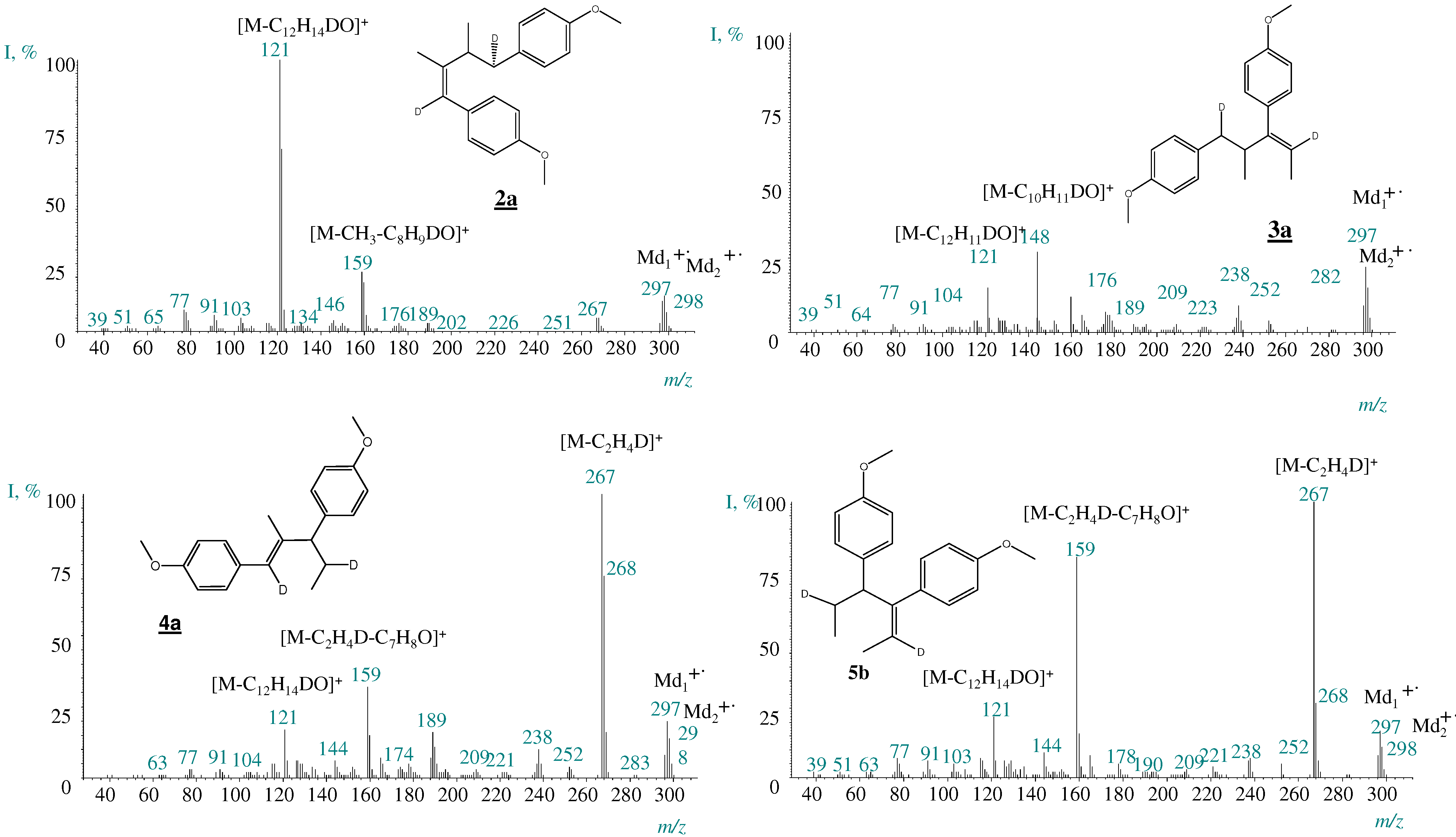
2.3. trans-Anethole phototransformation
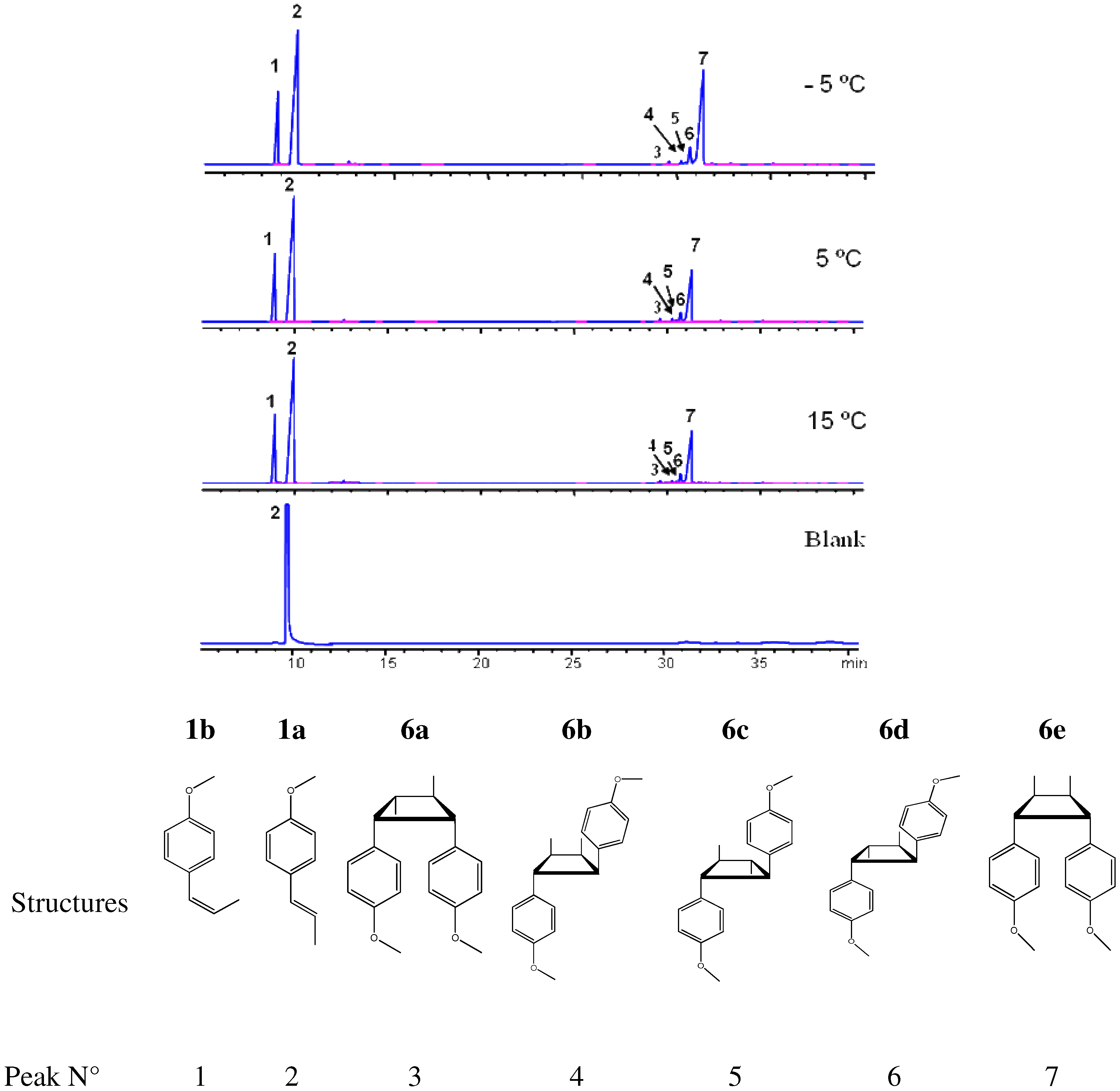
| Dimer | Molecular structure, R = p-Methoxy-phenyl | DB-5 column | tR, min (Polar column, DB-WAX) | Dipole moment, Debye | EI (eV) | |
|---|---|---|---|---|---|---|
| tR, min (Fig. 3) | RI | |||||
| 6a |  | 29.6 | 2139 | --- | 2.11 | 7.90 |
| 6b |  | 30.2 | 2106 | --- | 3.00 | 8.30 |
| 6c | 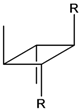 | 30.9 | 2266 | 142.8 | 2.54 | 8.10 |
| 6d | 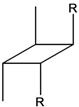 | 30.5 | 2289 | --- | 2.53 | 8.29 |
| 6e | 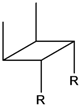 | 31.1 | 2255 | 151.4 | 2.37 | 8.27 |
| T ºC | trans- Anethole conversion, % | Selectivity, % | |||||
|---|---|---|---|---|---|---|---|
| cis-anethole | 6a | 6b | 6c | 6d | 6e | ||
| -5 | 30 | 42 | <1 | 2 | 5 | 1 | 50 |
| 5 | 35 | 38 | <1 | 2 | 5 | 2 | 54 |
| 15 | 52 | 34 | <1 | <1 | 8 | 1 | 57 |
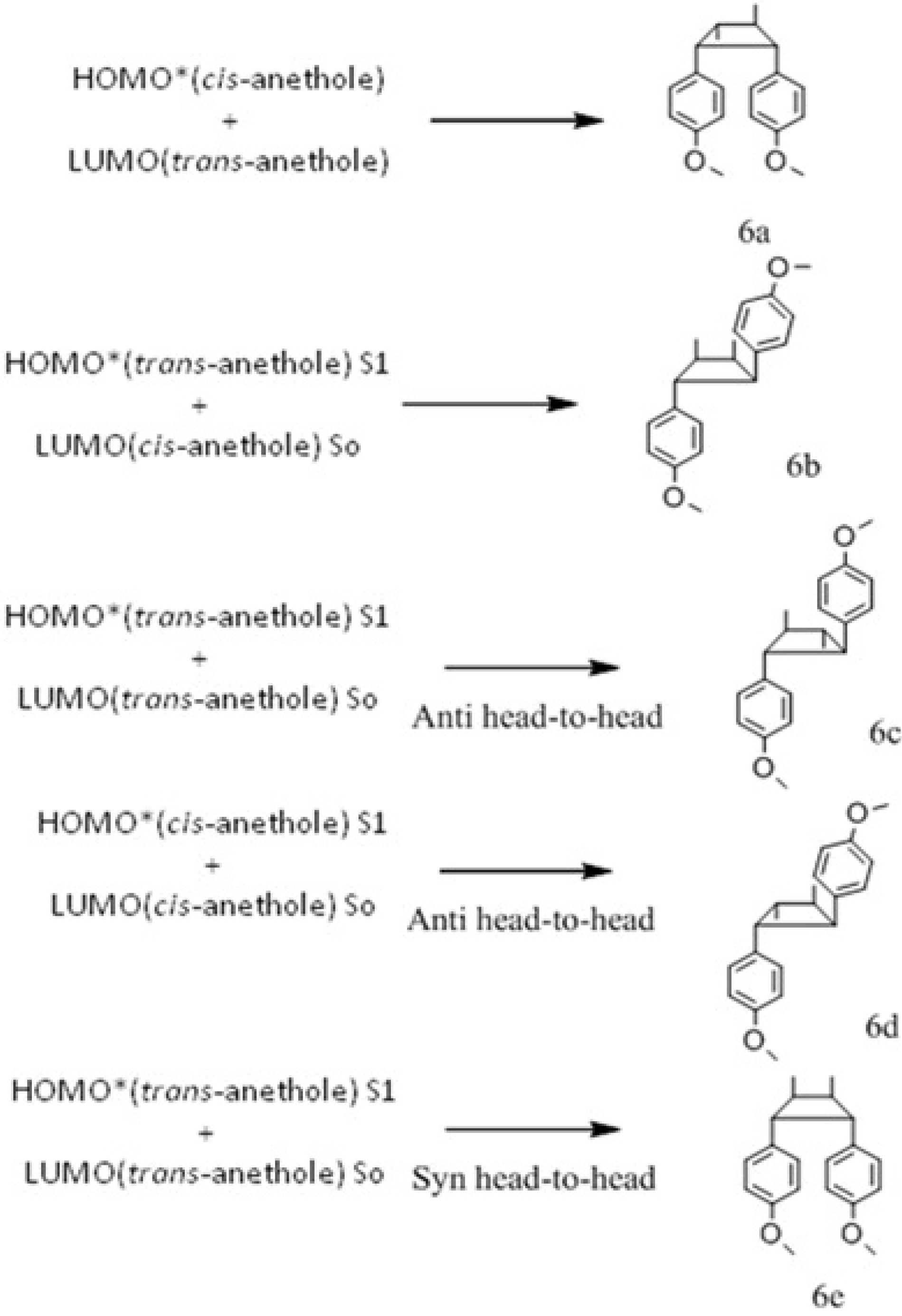
2.4. Quantum yield of trans-anethole photoisomerization
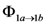 ) and 6c (
) and 6c ( 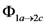 ) and 6e (
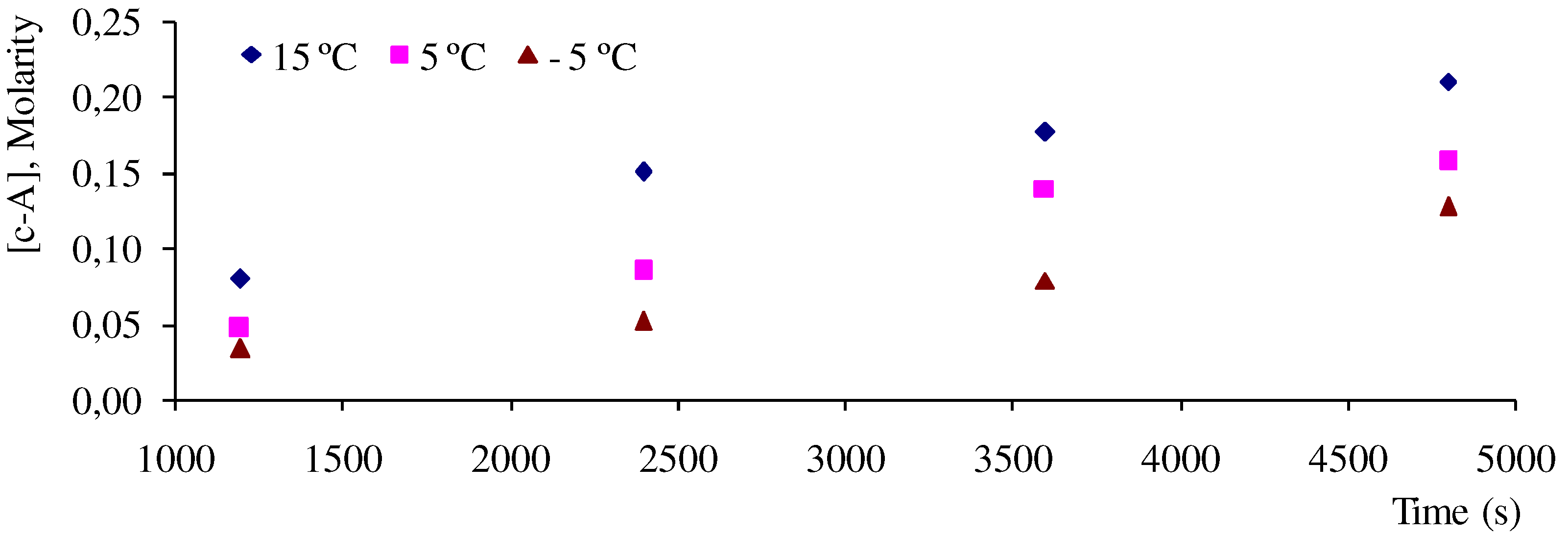
) and 6e (  ) dimers at ‑5, 5 and 15 °C, were calculated as the ratio of reaction rate over flux of absorbed photons (Table 4). In each case, the reaction rate was calculated as the slope of the plot of concentration Vs time. Figure 4 and Figure 5 show the linear nature of the concentration profiles of cis-anethole and dimers 6c and 6e. Potassium ferrioxalate solutions were employed in the actinometric determination of flux as 3 × 10‑4 ± 2 × 10-5 mol photon L-1 s-1.
) dimers at ‑5, 5 and 15 °C, were calculated as the ratio of reaction rate over flux of absorbed photons (Table 4). In each case, the reaction rate was calculated as the slope of the plot of concentration Vs time. Figure 4 and Figure 5 show the linear nature of the concentration profiles of cis-anethole and dimers 6c and 6e. Potassium ferrioxalate solutions were employed in the actinometric determination of flux as 3 × 10‑4 ± 2 × 10-5 mol photon L-1 s-1. 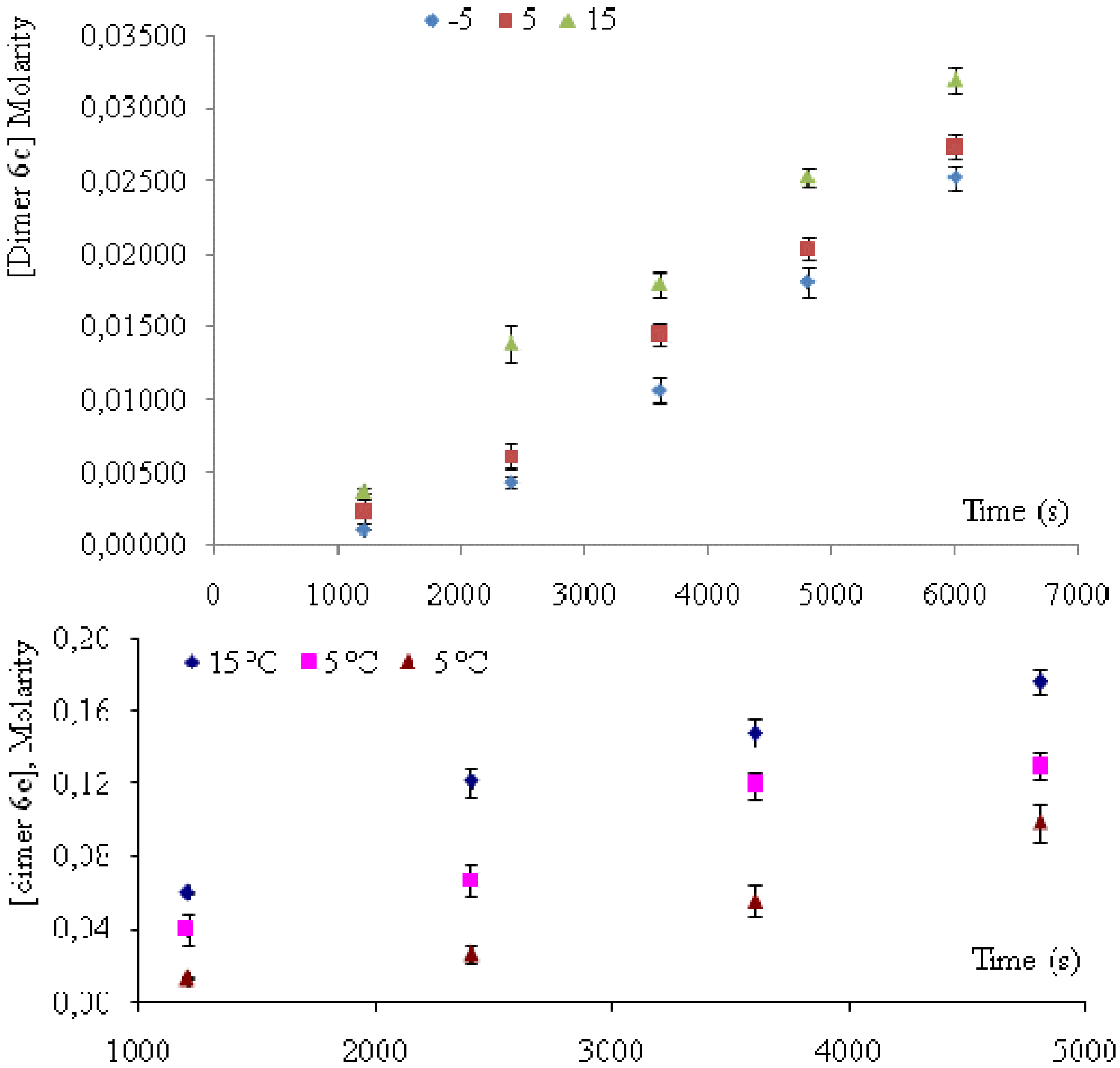
| T (ºC) | cis-Anethole a 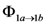 | 6c Dimer b 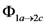 × 10 6 × 10 6 | 6e Dimer c  |
|---|---|---|---|
| -5 | 0.09 | 6 | 0.08 |
| +5 | 0.11 | 5 | 0.09 |
| +15 | 0.12 | 5 | 0.10 |
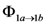 = 0.12 and 0.13, respectively) [26]. The increase of quantum yields with temperature indicates the existence of an energy barrier in photoisomerization and photodimerization. The
= 0.12 and 0.13, respectively) [26]. The increase of quantum yields with temperature indicates the existence of an energy barrier in photoisomerization and photodimerization. The 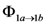 values determined after multichromatic UV-Vis irradiation of trans-anethole in toluene (0.35 M) are similar to the values reported for trans-anethole photoisomerization after monochromatic (254, 281, or 313 nm) irradiation [9,10]. Thus, there is no appreciable dependence of quantum yield on excitation wavelength. The quantum yield for trans-retinal photoisomerization (
values determined after multichromatic UV-Vis irradiation of trans-anethole in toluene (0.35 M) are similar to the values reported for trans-anethole photoisomerization after monochromatic (254, 281, or 313 nm) irradiation [9,10]. Thus, there is no appreciable dependence of quantum yield on excitation wavelength. The quantum yield for trans-retinal photoisomerization ( 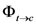 ~ 0.67) has been found to be independent of excitation wavelength [25]. On the other hand, conformational photoisomerization [26] of trans-stilbene and stilbene derivatives [27], some fluorinated compounds [28], and azobenzene and its dimers [29,30,31,32,33,34] has been reported as irradiation wavelength dependent.
~ 0.67) has been found to be independent of excitation wavelength [25]. On the other hand, conformational photoisomerization [26] of trans-stilbene and stilbene derivatives [27], some fluorinated compounds [28], and azobenzene and its dimers [29,30,31,32,33,34] has been reported as irradiation wavelength dependent.3. Experimental
3.1. General
3.2. Faujasite preparation and characterization
3.3. Zeolite characterization
3.4. trans-Anethole treatment with dealuminated HY zeolites
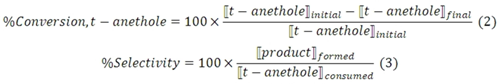

3.5. trans-Anethole treatment with deuterated HY zeolite
3.6. Phototransformation kinetics
3.7. Quantum yield determination

4. Conclusions
Acknowledgements
References
- Leung, A.Y.; Foster, S. Encyclopedia of Common Natural Ingredients: Used in Food, Drugs, and Cosmetics, 2nd ed; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 36–38. [Google Scholar]
- Pareck, S.K.; Trivedi, K.C.; Maheshwari, S.K.; Gangarade, S.K.; Gangarade, S.K.; Masheshwari, M.L.; Gupta, R. Studies on cultivation of anise in India. Indian Perfum. 1980, 24, 88–92. [Google Scholar]
- Cosentino, C.I.G.; Tuberodo, B.; Pisano, M.; Satta, V.; Mascia, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Newberne, P.; Smith, R.L.; Doull, J.; Goodman, J.I.; Munro, I.C.; Portoghese, P.S.; Wagner, B.M.; Weil, C.S.; Woods, L.A.; Adams, T.B.; Lucas, C.D.; Ford, R.A. The FEMA GRAS assessment of trans-anethole used as a flavoring substance. Food Chem. Toxicol. 1999, 37, 787–811. [Google Scholar]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Adams, T.B. Criteria for the safety evaluation of flavoring substances the expert panel of the flavor and extract manufacturers association. Food Chem. Toxicol. 2005, 43, 1141–1177. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, C.R.; Martínez, J.R.; Shibamoto, T. Catalytic transformation of anise oil (Pimpinellaanisum L.) over zeolite Y. J. High. Resolut. Chromatogr. 1995, 18, 501–503. [Google Scholar] [CrossRef]
- Biaglow, A.I.; Parrillo, D.J.; Kokotailo, G.T.; Gorte, R.J. A study of dealuminated faujasites. J. Catal. 1994, 148, 213–223. [Google Scholar]
- Weitkamp, J.; Puppe, L. Catalysis and Zeolites: Fundamentals and Applications; Springer: Heidelberg, Germany, 1999; p. 564. [Google Scholar]
- Lewis, F.D.; Kojima, M. Electron transfer induced photoisomerization, dimerization and oxygenation of trans-and-cis-anethole. The role of monomer and dimer cation radicals. J. Am. Chem. Soc. 1988, 110, 8664–8670. [Google Scholar]
- Lewis, F.D.; Kojima, M. Photodimerization of singlet trans-and-cis-anethole. Concerted or stepwise? J. Am. Chem. Soc. 1988, 110, 8660–8664. [Google Scholar] [CrossRef]
- Anand, P.; Maya, G; Tewari, S.C; Rastogi, S.N; Roy, S.K. Biological profile of 1,2-diethyl-1,3-bis-(p-methoxyphenyl)-1-propene—A new oral non-steroidal contraceptive. Indian J. Exp. Biol. 1980, 18, 557–560. [Google Scholar]
- Wessely, I.F.; Kerschbaum, E.; Kleedorfer, A.; Prillinger, F.; Zajic, E. Synthetic estrogens. Monatsh. Chem. 1940, 73, 127–158. [Google Scholar]
- Whitmore, F.C. Mechanism of the polymerization of olefins by acid catalysts. Ind. Eng. Chem. 1934, 26, 94–95. [Google Scholar] [CrossRef]
- Tung, S.E.; Mc. Ininch, E. Zeolitic aluminosilicate: I. Surface ionic diffusion, dynamic field, and catalytic activity with hexane on CaY. J. Catal. 1968, 10, 166–174. [Google Scholar]
- Docquir, F.; Norberg, V.; Toufar, H.; Paillaud, J.-L.; Su, B.L. Infrared study on the adsorption behavior of methylamine in a series of large pore cationic zeolites. A further confirmation of three types of interaction between methylamine and zeolites. Langmuir 2002, 18, 5963–5966. [Google Scholar]
- Janin, A.; Maache, M.; Lavalley, J.C.; Joly, J.F.; Raatz, F.; Szydlowski, N. FT IR study of the silanol groups in dealuminated HY zeolites-nature of the extraframework debris. Zeolites 1991, 11, 391–393. [Google Scholar] [CrossRef]
- Corma, A.; Planelles, J.; Sánchez-Marin, J.; Tomás, F. The nature of acids sites on fluorinated v-Al2O3. J. Catal. 1985, 92, 284–287. [Google Scholar]
- Corma, A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Haw, J.F.; Richardson, B.R.; Oshiro, I.S.; Lazo, N.D.; Speed, J.A. Reactions of propene on zeolite HY catalyst studied by in situ variable temperature solid-state nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1989, 111, 2052–2058. [Google Scholar] [CrossRef]
- Richardson, B.R.; Lazo, N.D.; Schettler, P.D.; White, J.L.; Haw, J.F. Reactions of butadiene in zeolite catalyst by in situ variable-temperature solid-state nuclear magnetic resonance spectrometry. J. Am. Chem. Soc. 1990, 112, 2886–2891. [Google Scholar] [CrossRef]
- Nozaki, H.; Otani, I.; Noyori, R.; Kawanisi, M. Photochemical reactions of trans-anethole. Tetrahedron 1968, 24, 2183–2192. [Google Scholar] [CrossRef]
- Meyer, S.; Metzger, J.O. Use of electrospray ionization mass spectrometry for the investigation of radical catión chain reactions in solution: detection of transient radical cations. Anal. Bioanal. Chem. 2003, 377, 1108–1114. [Google Scholar] [CrossRef]
- Marquez, C.A.; Wang, H.; Fabbretti, F.; Metzger, J.O. Electron-transfer-catalyzed dimerization of trans-anethole: Detection of distonictetramethylene radical cation intermediate by extractive electrospray ionization mass spectrometry. J. Am. Chem. Soc. 2008, 130, 17208–17209. [Google Scholar] [CrossRef]
- Lewis, F.D.; Bassani, D.M.; Caldwell, R.A.; Unett, D.J. Singlet state cis, trans photoisomerization and intersystem crossing of 1-arylpropenes. J. Am. Chem. Soc. 1994, 116, 10477–10485. [Google Scholar]
- Kim, J.E.; Tauber, M.J.; Mathies, R.A. Wavelength dependent cis-trans isomerization in vision. Biochemistry 2001, 40, 13774–13778. [Google Scholar] [CrossRef]
- Petterson, M.; Maçôas, E.M.S.; Khriachtechev, L.; Fausto, R.; Räsänen, M. Conformational isomerization of formic acid by vibrational excitation at energies below the torsional barrier. J. Am. Chem. Soc. 2003, 125, 4058–4059. [Google Scholar] [CrossRef]
- Seydack, M.; Bending, J. The anomalous excited-state temperature behavior of trans-4,4-diaminostilbene and trans-4,4-di(phenyl-ureanyl)-stilbene. J. Phys. Chem. A 2001, 105, 5731–5733. [Google Scholar] [CrossRef]
- Barr, J.W.; Bell, T.W.; Catalano, V.J.; Cline, J.I.; Phillips, D.J.; Procupez, R. Syntheses, structures, and photoisomerization of (E)- and (Z) -2- tert-butyl-9-(2,2,2,-triphenyl-ethylidene)fluorine. J. Phys. Chem. A 2005, 109, 11650–11654. [Google Scholar]
- Arai, T.; Sakuragi, H.; Tokumaru, K. Photosensitized cis-trans isomerization of β-alkylstyrenes. B. Chem. Soc. Japn. 1982, 55, 2204–2206. [Google Scholar] [CrossRef]
- Norikane, Y.; Tamaoki, N. Photochemical and thermal cis-/trans-isomerization of cyclic and noncyclicazobenzene dimmers: effect of a cyclic structure on isomerization. Eur. J. Org. Chem. 2006, 1, 1296–1302. [Google Scholar] [CrossRef]
- Ronayette, J.; Arnaud, R.; Lebourgeois, P.; Lemaire, J. Photochemical isomerization of azobenzene in solution. Can. J. Chem. 1974, 52, 1848–1857. [Google Scholar] [CrossRef]
- Schultz, T.; Quenneville, J.; Levine, B.; Toniolo, A.; Martínez, T.J.; Lochbrunner, S.; Schmitt, M.; Shaffer, J.P.; Zgierski, M.Z.; Stolow, A. Mechanism and dynamics of azobenzene photoisomerization. J. Am. Chem. Soc. 2003, 125, 8098–8099. [Google Scholar]
- Yamashita, S.; Ono, H.; Toyama, O. The cis-trans photoisomerization of azobenzene. B. Chem. Soc. Japn. 1962, 35, 1849–1853. [Google Scholar] [CrossRef]
- Zimmermann, G.; Ghow, L.Y.; Paik, U.J. The photochemical isomerization of azobenzene. J. Am. Chem. Soc. 1958, 80, 3528–3531. [Google Scholar] [CrossRef]
- Kuhn, H.J.; Braslavsky, S.E.; Schmidt, R. Chemical Actinometry. IUPAC Technical Report. Pure Appl. Chem. 2004, 76, 2105–2146. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Castro, H.T.; Martínez, J.R.; Stashenko, E. Anethole Isomerization and Dimerization Induced by Acid Sites or UV Irradiation. Molecules 2010, 15, 5012-5030. https://doi.org/10.3390/molecules15075012
Castro HT, Martínez JR, Stashenko E. Anethole Isomerization and Dimerization Induced by Acid Sites or UV Irradiation. Molecules. 2010; 15(7):5012-5030. https://doi.org/10.3390/molecules15075012
Chicago/Turabian StyleCastro, Hans T., Jairo René Martínez, and Elena Stashenko. 2010. "Anethole Isomerization and Dimerization Induced by Acid Sites or UV Irradiation" Molecules 15, no. 7: 5012-5030. https://doi.org/10.3390/molecules15075012
APA StyleCastro, H. T., Martínez, J. R., & Stashenko, E. (2010). Anethole Isomerization and Dimerization Induced by Acid Sites or UV Irradiation. Molecules, 15(7), 5012-5030. https://doi.org/10.3390/molecules15075012