Microwave Synthesis, Basic Spectral and Biological Evaluation of Some Copper (II) Mesoporphyrinic Complexes
Abstract
:1. Introduction

2. Results and Discussion
2.1. Infrared spectra
| Characteristic vibration | Wavenumber of the IR band (cm-1) | |||
|---|---|---|---|---|
| TCMP1 | Cu(II)TCMP | TCMPOHm1 | Cu(II) TCMPOHm | |
| νO-H | - | - | 3503 m | 3493 m |
| νN-H | 3310 w | - | 3312 m | - |
| νC-H | 2950 m | 2954 m | 2952 m | 2957 m |
| νC-H | 2923 m | 2924 m | 2923 v.s | 2923 m |
| νC-H from -O-CH3 | 2851 s | 2853 s | 2853 m | 2848 s |
| νC=O | 1717 v.s. | 1715 s | 1718 s | 1720 m |
| νC-N | 1603 m | 1604 m | 1597 s | 1600 s |
| νC-H pyrrole | 1400 m | 1402 w | 1401 m | 1410 w |
| νC-O | 1156 s | 1164 m | 1156 s | 1164 m |
| δC-H | 1018 m | 1014 m | 1020 w | 1010 w |
| δN-H pyrrole | 963 m | - | 964 m | - |
| γC-C | 869 w | 858 w | 862 w | |
| γC-N pyrrole | 798 m | 797 s | 790 m | 795 s |
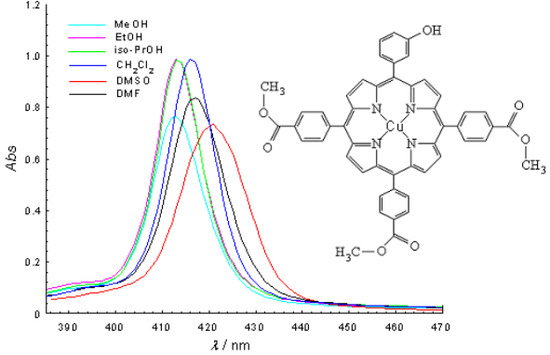
2.2. Molecular electronic spectra


| Solvent | λmax (nm) [lgε (L mol-1 cm-1)] | ||||
|---|---|---|---|---|---|
| Soret B(0,0) | Qy(1,0) | Qy(0,0) | Qx(1,0) | Qx(0,0) | |
| 5, 10, 15, 20-meso-tetrakis-(4-carboxymethylphenyl) - 21,23-H porphine | |||||
| MeOH | 415.3 [5.528] | 514.8 [4.415] | 544.3[4.387] | 591.9[4.265] | 646.9 [4.342] |
| EtOH | 416.4 [5.546] | 512.5 [4.428] | 547.4 [4.283] | 590.4 [4.225] | 647.4 [4.146] |
| iso-PrOH | 416.8 [5.511] | 513.5 [4.301] | 546.9 [4.049] | 592.4 [3.857] | 648.4 [3.602] |
| CH2Cl2 | 419.6 [5.699] | 515.2 [4.344] | 550.0 [4.000] | 590.1 [3.806] | 645.5 [3.643] |
| DMSO | 420.9 [5.602] | 515.6 [4.310] | 550.0 [4.049] | 590.1 [4.000] | 645.6 [3.833] |
| DMF | 419.3 [5.662] | 514.3 [4.326] | 548.7 [4.017] | 589.6 [3.833] | 645.3 [3.716] |
| 5, 10 ,15 ,20–meso-tetrakis-(4-carboxymethylphenyl)-21,23-Cu(II)porphine | |||||
| MeOH | 412.5 [5.637] | - | 538.2 [4.024] | - | - |
| EtOH | 413.0 [5.709] | - | 537.7 [4.107] | - | - |
| iso-PrOH | 413.1 [5.527] | - | 537.0 [4.274] | 574.8(sh) | - |
| CH2Cl2 | 416.4 [5.697] | - | 539.4 [4.387] | 574.5(sh) | - |
| DMSO | 421.6 [5.582] | - | 544.1 [4.334] | 584.6 [3.681] | - |
| DMF | 417.0 [5.627] | - | 540.3 [4.310] | 578.0 (sh) | - |
| 5- (3-hydroxyphenyl)-10, 15, 20–tris-(4-carboxymethylphenyl) – 21,23-H porphine | |||||
| MeOH | 422.4 [5.582] | 513.1 [4.158] | 554.8 [4.193] | 595.5 [3.924] | 647.6 [3.643] |
| EtOH | 424.1 [5.561] | 513.4 [4.134] | 556.3 [4.158] | 596.1 [3.857] | 649.4 [3.556] |
| iso-PrOH | 424.6 [5.577] | 513.1 [4.146] | 556.0 [4.182] | 596.7 [3.881 | 650.3 [3.602] |
| CH2Cl2 | 420.1 [5.607] | 515.2 [4.121] | 548.8 [4.146] | 588.9 [3.716] | 647.9 [3.556] |
| DMSO | 429.3 [5.546] | 516.1 [4.170] | 558.6 [4.182] | 598.8 [3.944] | 648.8 [3.681] |
| DMF | 426.5 [5.519] | 514.9 [4.107] | 556.8 [4.121] | 597.9 [3.833] | 648.2 [3,556] |
| 5-(3-hydroxyphenyl)-10, 15, 20-tris-(4-carboxymethylphenyl)-21,23- Cu(II)porphine | |||||
| MeOH | 412.7 [5.486] | - | 538.3 [4.255] | - | - |
| EtOH | 413.1 [5.598] | - | 537.1 [4.318] | - | - |
| iso-PrOH | 413.6 [5.593] | - | 537.7 [4.342] | 570.3(sh) | - |
| CH2Cl2 | 416.1 [5.598] | - | 539.5 [4.310] | - | - |
| DMSO | 421.0 [5.468] | - | 544.0 [4.265] | 585.9(sh) | - |
| DMF | 417.0 [5.525] | - | 540.0 [4.283] | 579.6(sh) | - |
2.3. EPR spectra
| Complex combination | g|| | g⊥ | A|| x 104 (cm-1) | A⊥ x 104 (cm-1) |
|---|---|---|---|---|
| Cu(II)TPPOHm1 | 2.113 | 2.055 | 202 | 28 |
| Cu(II)TCMP | 2.162 | 2.042 | 203 | 32 |
| Cu(II)TCMPOHm | 2.170 | 2.045 | 201 | 33 |
2.4. Preliminary toxicological tests


3. Experimental
3.1. Materials and Methods
3.2. Synthesis of copper porphyrinic complexes
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Biesaga, M.; Pyrzyńska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Moan, J.; Peng, Q. An outline of history of PDT. In Photodynamic Therapy; Patrice, T., Ed.; Royal Society of Chemistry: Cambridge, UK, 2004; pp. 1–18. [Google Scholar]
- Stockert, J.C.; Cañete, M.; Juarranz, A.; Villanueva, A.; Horobin, R.W.; Borrell, J.I.; Teixidó, J.; Nonell, S. Porphycenes: Facts and Prospects in Photodynamic Therapy of Cancer. Curr. Med. Chem. 2007, 14, 997–1026. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Caprioli, S.; Mazzagatti, L.; Canti, G.; Ravizza, R.; Gariboldia, M.; Montia, E. Photodynamic effects of porphyrin and chlorin photosensitizers in human colon adenocarcinoma cells. Bioorg. Med. Chem. 2004, 12, 4853–4860. [Google Scholar] [CrossRef]
- Chen, J.Y.; Mak, N.K.; Yow, C.M.N.; Fung, M.C.; Chiu, L.C.; Leung, W.N.; Cheung, N.H. The Binding Characteristics and Intracellular Localization of Temoporfin (mTHPC) in Myeloid Leukemia Cells: Phototoxicity and Mitochondrial Damage. Photochem. Photobiol. 2000, 72, 541–547. [Google Scholar] [CrossRef]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3195. [Google Scholar] [CrossRef]
- Grosseweiner, L.I. The Science of Phototherapy; CRC Press: London, UK, 1994; p. 139. [Google Scholar]
- Schweiter, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Delivery Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Mody, T.D.; Sessler, J.L. Porphyrin- and Expanded Porphyrin- based Diagnostic and Therapeutic Agents In Supramolecular Materials and Technologies; Reinhoud, D.N., Ed.; Wiley: Chichester, UK, 1999; Volume 4, p. 126. [Google Scholar]
- Kreimer-Birnbaum, M. Modified porphyrins, chlorins, phthalocyanines, and purpurins: second-generation photosensitizers for photodynamic therary. Semin. Hematol. 1989, 26, 157–173. [Google Scholar]
- Kostenich, G.; Orenstein, A.; Malik, Z.; Ehrenberg, B. Preclinical photodynamic therapy studies with endogenous and new exogenous photosensitizers. In Quantitative Data of 2nd and 3rd Generation Photosensitizers for Photodynamic Therapy; Moser, J.G., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1998; pp. 101–114. [Google Scholar]
- Ris, H.B.; Krueger, T.; Giger, A.; Lim, C.K; Stuart, J.C.M; Althaus, U.; Altermatt, H.J. Photodynamic therapy with mTHPC and polyethylene glycol-derived mTHPC: a comparative study on human tumour xenografts. Br. J. Cancer 1999, 79, 1061–1066. [Google Scholar] [CrossRef]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy (Advanced Chemistry Texts V.1); Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 2000; pp. 57–112. [Google Scholar]
- Postino, F.; Mora, M.; DeMadariaga, M.A.; Nonell, S.; Sagrista, M.L. Incorporation of hydrophobic porphyrins into liposomes: characterization and structural requirements. Int. J. Pharma. 2004, 278, 239–254. [Google Scholar] [CrossRef]
- Scalise, I.; Durantini, E.N. Photodynamic effect of metallo 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl) porphyrins in biomimetic AOT reverse micelles containing urease. J. Photochem. Photobiol. A 2004, 162, 105–113. [Google Scholar] [CrossRef]
- Boyle, R.B.; Dolphin, D. Structure and Biodistribution Relationships of Photodynamic Sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef]
- Mac Donald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyrins Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B 2004, 73, 1–28. [Google Scholar] [CrossRef]
- Ricchelli, F.; Jori, G.; Gobbo, S.; Tronchin, M. Liposomes as models to study the distribution of porphyrins in cell membranes. Biochim. Biophys. Acta 1991, 1065, 42–48. [Google Scholar]
- J. Osterloh, M.G.H. Vicente, Mechanisms of porphyrinoid localization in tumors. J. Porphyrins Phthalocyanines 2002, 6, 305–325. [Google Scholar] [CrossRef]
- Milgrom, L.; MacRobert, S. Light years ahead. Chem. Br. 1998, 34, 45–50. [Google Scholar]
- Socoteanu, R.; Boscencu, R.; Nacea, V.; Sousa Oliveira, A.; Vieira Ferreira, L.F. Microwave-assisted Synthesis of Unsymmetrical Tetrapyrrolic Compounds. Rev. Chim. 2008, 59, 969–972. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Vieira Ferreira, L.F.; Nacea, V.; Patrinoiu, G. Synthesis and Characterization of Some Unsymmetrically-substituted Mesoporphyrinic Mono-Hydroxyphenyl Complexes of Copper(II). Pol. J. Chem. 2008, 82, 509–522. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Ferreira, L.F.V. Studies on Zn(II) monohydroxyphenyl mesoporphyrinic complexes. Synthesis and characterization. J. Serb. Chem. Soc. 2008, 73, 713–726. [Google Scholar] [CrossRef]
- Milgrom, L.R. The Colours of Life. An Introduction to the Chemistry of Porphyrins and Related Compounds; Oxford University Press: Oxford, UK, 1977; pp. 1–3. [Google Scholar]
- Harriman, A. Luminescence of porphyrins and metalloporphyrins. Part 2. Copper(II), chromium(III), manganese(III), iron(II) and iron(III) porphyrins. J. Chem. Soc. Faraday Trans. 1981, 77, 369–377. [Google Scholar] [CrossRef]
- Reichardt, C.H. Solvents and Solvent Effects in Organic Chemistry; VCH: New York, NY, USA, 1988. [Google Scholar]
- Lin, W.C. Electron Spin Resonance and Electronic Structure of Metalloporphyrins. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, USA, 1978; Volume 4, p. 358. [Google Scholar]
- Manoharan, P.T.; Roger, M.T. Electron Spin Resonance of Metal Complexes; Yen, T.F., Ed.; Plenum Press: New York, NY, USA, 1969; p. 143. [Google Scholar]
- Kivelson, D.; Neiman, R.R. ESR Studies on the Bonding in Copper Complexes. J. Chem. Phys. 1961, 35, 149–155. [Google Scholar] [CrossRef]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Meth. 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar] [CrossRef]
- Adam, D. Microwave chemistry: Out of the kitchen. Nature 2003, 421, 571–572. [Google Scholar] [CrossRef]
- Wei, T.H. Microwave-assisted synthesis and reverse saturable absorption of phthalocyanines and porphyrins. J. Organomet. Chem. 2004, 689, 1078–1084. [Google Scholar] [CrossRef]
- Liu, M.O.; Hu, A.T. Microwave-assisted synthesis of phthalocyanine–porphyrin complex and its photoelectric conversion properties. J. Organomet. Chem. 2004, 689, 2450–2455. [Google Scholar] [CrossRef]
- Boufatah, N.; Gellis, A.; Maldonado, J.; Vanelle, P. Efficient microwave-assisted synthesis of new sulfonylbenzimidazole-4,7-diones: heterocyclic quinones with potential antitumor activity. Tetrahedron 2004, 60, 9131–9137. [Google Scholar] [CrossRef]
- Sauer, D.R.; Kalvin, D.; Phelan, K.M. Microwave-Assisted Synthesis Utilizing Supported Reagents: A Rapid and Efficient Acylation Procedure. Org. Lett. 2003, 5, 4721–4724. [Google Scholar] [CrossRef]
- Yoon, D.S.; Han, Y.; Stark, T.M.; Haber, J.C.; Gregg, B.T.; Stankovich, S.B. Efficient Synthesis of 4-Aminoquinazoline and Thieno[3,2-d]pyrimidin-4-ylamine Derivatives by Microwave Irradiation. Org. Lett. 2004, 6, 4775–4778. [Google Scholar]
© 2010 by the authors;
Share and Cite
Boscencu, R.; Ilie, M.; Socoteanu, R.; Oliveira, A.S.; Constantin, C.; Neagu, M.; Manda, G.; Ferreira, L.F.V. Microwave Synthesis, Basic Spectral and Biological Evaluation of Some Copper (II) Mesoporphyrinic Complexes. Molecules 2010, 15, 3731-3743. https://doi.org/10.3390/molecules15053731
Boscencu R, Ilie M, Socoteanu R, Oliveira AS, Constantin C, Neagu M, Manda G, Ferreira LFV. Microwave Synthesis, Basic Spectral and Biological Evaluation of Some Copper (II) Mesoporphyrinic Complexes. Molecules. 2010; 15(5):3731-3743. https://doi.org/10.3390/molecules15053731
Chicago/Turabian StyleBoscencu, Rica, Mihaela Ilie, Radu Socoteanu, Anabela Sousa Oliveira, Carolina Constantin, Monica Neagu, Gina Manda, and Luis Filipe Vieira Ferreira. 2010. "Microwave Synthesis, Basic Spectral and Biological Evaluation of Some Copper (II) Mesoporphyrinic Complexes" Molecules 15, no. 5: 3731-3743. https://doi.org/10.3390/molecules15053731