Antioxidant Capacity of Macaronesian Traditional Medicinal Plants
Abstract
:1. Introduction
| Family | Plant name | Use in folk medicine |
|---|---|---|
| Lauraceae | Ocotea foetens (Ainton) Baill | O. foetens is used as antihypertensive, treatment for malignant diseases (poultices) and cancer [10,11] |
| Apollonias barbujana (Cav.) Bornm | Plants of this family have been purported in folk medicine as diuretic, analgesic, antiulcerogenic, cytostatic, cardiotonic, expectorant, stomachic, sedative or carminative effects and against rheumatic pain [12] | |
| Polygonaceae | Rumex maderensis Lowe | R. maderensis infusion is used as a diuretic and blood depurative and externally applied in poultices for dermatosis [11,13] Rumex sp. are used to treat headaches, to promote maturation abscess, in wound healing, for infected wounds and pimples [14,15] |
| Plantaginaceae | Plantago arborescens Poir. subsp. maderensis (Decne.) A. Hans. et Hunk. | Plantago sp. is used in infusion gargled to relieve sore throat, on the treatment of hepatitis, conjunctivitis, furunculosis, diarrhoea, malignant diseases, spasm, intestinal and stomach ulcers, stomach ache, tuberculosis, asthma, cough, bronchitis, boils, diabetes, goiter, it is also used as hemostatic, antitussive and expectorant and applied externally as a poultice to treat wounds, cuts and bee bites [10,11,15,16,17,18,19] |
| Rosaceae | Prunus azorica (Mouill.) Rivas Mart., Lousã, Fern.Prieto, E.Días, J.C.Costa & C.Aguiar | Prunus spp. are used in treatment of urinary tract diseases [16,18,19] |
2. Results and Discussion
2.1. Total phenol and flavonoid content

| Plant species | Total phenols (TP) (mg GAE g-1 dw) | Total flavonoids (TF) (mg CE g-1 dw) |
|---|---|---|
| A. barbujana | 35.8 ± 4.4 a | 18.31 ± 2.51 a |
| O. foetens | 10.9 ± 0.8 bc | 5.03 ± 0.42 c |
| P. azorica | 15.4 ± 0.8 b | 11.29 ± 0.21 b |
| P. arborescens subsp. maderensis | 5.4 ± 1.1 c | 0.22 ± 0.02 d |
| R. maderensis | 9.9 ± 1.6 bc | 5.23 ± 0.10 c |
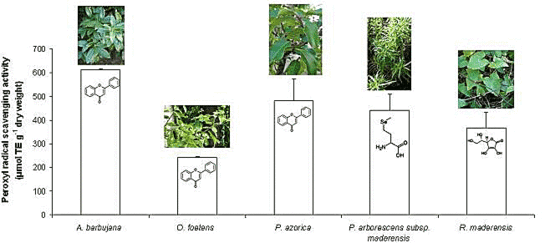
2.2. Free radical scavenging activity


2.3. HPLC profile


2.4. Trace elements and free amino acids
| Plant species | Cu (µg g-1 dw) | Fe (µg g-1 dw) | Mn (µg g-1 dw) | Se (µg g-1 dw) | Zn (µg g-1 dw) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Determined values | Reference values | Determined values | Reference values | Determined values | Reference values | Determined values | Reference values | Determined values | Reference values | |
| A. barbujana | 4.09 | 59.91 | 63.03 | 0.07 | 14.26 | |||||
| O. foetens | 12.43 | 34.40 | 12.34 | 0.13 | 14.47 | |||||
| P. azorica | 6.87 | 4.8-23.8 a,b,g,h | 66.81 | 25.18-1117 h,i,j,k | 16.65 | 1.8-74.8 a ,b,g,h | 0.07 | 0.003-0.088 g,h | 11.99 | 1.8-49.8 a ,b,g,h |
| P. arborescens subsp. maderensis | 13.58 | 15.2-41.1 e | 160.64 | 27.17 | 80.5-229 e | 0.26 | 0.2 f | 46.08 | 36.5-71.8 e | |
| R. maderensis | 9.75 | 19-39 c,d | 190.68 | 241-425 h | 29.20 | 0.13 | 47.71 | 37-120 c,d | ||
| Free amino acid | Molecular weight | Content (μg g-1 dw) |
|---|---|---|
| Hydroxyproline | 131.1 | 253.60 |
| Serine | 105.1 | 23.11 |
| Asparagine | 132.1 | 83.35 |
| Proline | 115.4 | 105.40 |
| Se-Cysteine | 182.08 | 122.60 |
| Valine | 117.2 | 152.72 |
| Se-Methionine | 196.11 | 414.35 |
| Free amino acid | Concentration (mg mL-1) | Peroxyl radical scavenging activity (TE) |
|---|---|---|
| Se-Cysteine | 0.0024 | 0.63 ± 0.13 |
| Se-Methionine | 0.0080 | 12.90 ± 2.15 |
3. Experimental
3.1. Plant material
3.2. Extraction procedure
3.3. Total phenolic content
3.4. Total flavonoid content
3.5. Free radical scavenging assays
3.5.1. Peroxyl radical scavenging capacity
3.5.2. Hydroxyl radical scavenging assay
3.6. HPLC profile of phenolic compounds
3.7. Quantification of L-ascorbic acid
3.8. Mineral composition
3.9. HPLC quantification of free amino acids
3.10. Statistical analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721. [Google Scholar] [CrossRef]
- Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003, 523-524, 9–20. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Riceevans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic. Biol. Med. 1995, 19, 481–486. [Google Scholar] [CrossRef]
- Esposito, E.; Rotilio, D.; Di Matteo, V.; Di Giulio, C.; Cacchio, M.; Algeri, S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging 2002, 23, 719–735. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Aspects Med. 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Branco, A.F. A flora madeirense na medicina popular. Separata da Revista "Brotéria" 1935, IV, 35–46. [Google Scholar]
- Rivera, D.; Obon, C. The ethnopharmacology of Madeira and Porto-Santo islands, a review. J. Ethnopharmacol. 1995, 46, 73–93. [Google Scholar] [CrossRef]
- Perez, C.; Trujillo, J.M.; Almonacid, L.N.; Navarro, E.; Alonso, S.J. New C13-norisoprenoids from Apollonias barbujana. Nat. Prod. Lett. 1996, 8, 1–6. [Google Scholar] [CrossRef]
- Ballabio, R. Plantes médicinales endémiques de l'île de Madère. Phytothérapie 2004, 2, 41–46. [Google Scholar]
- Kupeli, E.; Orhan, I.; Yesilada, E. Evaluation of some plants used in Turkish folk medicine for their anti-inflammatory and antinociceptive activities. Pharm. Biol. 2007, 45, 547–555. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef]
- Santos, S.; Correia, A.I.D.; Figueiredo, A.C.; Dias, L.S.; Dias, A.S. Plantas medicinais da península de Setúbal. Contribuição para o conhecimento da sua relevância etnobotânica. In Potencialidades e aplicações das plantas aromáticas e medicinais. Curso teórico-prático; Figueiredo, A.C., Barroso, J.G., Pedro, L.G., Eds.; Faculdade de Ciências da Faculdade de Ciências da Universidade de Lisboa - Centro de Biotecnologia Vegetal: Lisboa, Portugal, 2007; pp. 175–182. [Google Scholar]
- Sequeira, M.M.; Fontinha, S.M.G.S.V.; Freitas, F.I.C.; Ramos, L.C.; Mateus, M.G.H. Plantas e usos tradicionais nas memórias de hoje; Casa do Povo da Ilha/ Parque Natural da Madeira: Funchal, Portugal, 2006. [Google Scholar]
- World Health Organization, WHO Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 1999.
- World Health Organization, WHO Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 2007.
- Tavares, L.; Fortalezas, S.; Carrilho, D.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C. Antioxidant and antiproliferative properties of strawberry tree tissues. J. Berry Res. 2010. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D.J. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar]
- Atala, E.; Vásquez, L.; Speisky, H.; Lissi, E.; López-Alarcón, C. Ascorbic acid contribution to ORAC values in berry extracts: An evaluation by the ORAC-pyrogallol red methodology. Food Chem. 2009, 113, 331–335. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Robards, K.; Antolovich, M. Analytical chemistry of fruit bioflavonoids - A review. Analyst 1997, 122, R11–R34. [Google Scholar] [CrossRef]
- Ozcan, M. Mineral contents of some plants used as condiments in Turkey. Food Chem. 2004, 84, 437–440. [Google Scholar] [CrossRef]
- Jimenez, S.; Pinochet, J.; Gogorcena, Y.; Betran, J.A.; Moreno, M.A. Influence of different vigour cherry rootstocks on leaves and shoots mineral composition. Sci. Hortic. 2007, 112, 73–79. [Google Scholar]
- Alfawaz, M.A. Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J. Food Comp. Anal. 2006, 19, 552–555. [Google Scholar] [CrossRef]
- Shun-xing, L.; Nan-sheng, D.; Feng-ying, Z. Effect of digestive site acidity and compatibility on the species, lipopily and bioavailability of iron, manganese and zinc in Prunus persica Batsch and Carthamus tinctorus. Bioorg. Med. Chem. Lett. 2004, 14, 505–510. [Google Scholar] [CrossRef]
- Del Rio, M.; Font, R.; Almela, C.; Velez, D.; Montoro, R.; Bailon, A.D. Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcollar mine. J. Biotechnol. 2002, 98, 125–137. [Google Scholar]
- Lazaro, J.D.; Kidd, P.S.; Martinez, C.M. A phytogeochernical study of the Tras-os-Montes region (NE Portugal): Possible species for plant-based soil rernediation technologies. Sci. Total Environ. 2006, 354, 265–277. [Google Scholar] [CrossRef]
- von Boberfeld, W. Effect of selenium containing multi-nutrient fertilizer on selenium and sulphur concentrations depending on main species, growth, and dosage. Pflanzenbauwissenschaften-German J. Agron. 2002, 6, 84–92. [Google Scholar]
- Calisir, S.; Haciseferogullari, H.; Ozcan, M.; Arslan, D. Some nutritional and technological properties of wild plum (Prunus spp.) fruits in Turkey. J. Food Eng. 2005, 66, 233–237. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Gomez-Aparisi, J.; Betran, J.A.; Moreno, M.A. Influence of almond x peach hybrids rootstocks on flower and leaf mineral concentration, yield and vigour of two peach cultivars. Sci. Hortic. 2005, 106, 502–514. [Google Scholar] [CrossRef]
- Moodley, R.; Kindness, A.; Jonnalagadda, S.B. Elemental composition and chemical characteristics of five edible nuts (almond, Brazil, pecan, macadamia and walnut) consumed in Southern Africa. J. Environ. Sci. Health B 2007, 42, 585–591. [Google Scholar] [CrossRef]
- Finley, J.W. Selenium accumulation in plant foods. Nutr. Rev. 2005, 63, 196–202. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Assmann, A.; Bonifacic, M.; Briviba, K.; Sies, H.; Asmus, K.-D. One-electron reduction of selenomethionine oxide. Free Radical. Res. 2000, 32, 371–376. [Google Scholar] [CrossRef]
- Padmaja, S.; Squadrito, G.L.; Lemercier, J.N.; Cueto, R.; Pryor, W.A. Rapid oxidation of -selenomethionine by peroxynitrite. Free Radic. Biol. Med. 1996, 21, 317–322. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Mishra, B. Radical cations of some water-soluble organoselenium compounds: Insights from pulse radiolysis studies. Radiat. Phys. Chem. 2008, 77, 1294–1299. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Michalska, A.; Ceglinska, A.; Zielinski, H. Bioactive compounds in rye flours with different extraction rates. Eur. Food Res. Technol. 2007, 225, 545–551. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Bravo, M.N.; Silva, S.; Coelho, A.V.; Boas, L.V.; Bronze, M.R. Analysis of phenolic compounds in Muscatel wines produced in Portugal. Anal. Chem. Acta 2006, 563, 84–92. [Google Scholar] [CrossRef]
- Cohen, S.A.; Bidlingmeyer, B.A.; Tarvin, T.L. PITC derivatives in amino acid analysis. Nature 1986, 320, 769–770. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tavares, L.; Carrilho, D.; Tyagi, M.; Barata, D.; Serra, A.T.; Duarte, C.M.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R.; Chicau, P.; et al. Antioxidant Capacity of Macaronesian Traditional Medicinal Plants. Molecules 2010, 15, 2576-2592. https://doi.org/10.3390/molecules15042576
Tavares L, Carrilho D, Tyagi M, Barata D, Serra AT, Duarte CMM, Duarte RO, Feliciano RP, Bronze MR, Chicau P, et al. Antioxidant Capacity of Macaronesian Traditional Medicinal Plants. Molecules. 2010; 15(4):2576-2592. https://doi.org/10.3390/molecules15042576
Chicago/Turabian StyleTavares, Lucélia, Dina Carrilho, Meenu Tyagi, David Barata, Ana Teresa Serra, Catarina Maria Martins Duarte, Rui Oliveira Duarte, Rodrigo Pedro Feliciano, Maria Rosário Bronze, Paula Chicau, and et al. 2010. "Antioxidant Capacity of Macaronesian Traditional Medicinal Plants" Molecules 15, no. 4: 2576-2592. https://doi.org/10.3390/molecules15042576