Different Anthocyanin Profiles of the Skin and the Pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) Grape Berries
Abstract
:1. Introduction
2. Results and Discussion
2.1. 3’-Substituted anthocyanins and 3’,5’-substituted anthocyanins
2.2. Methoxylated anthocyanins
2.3. Acylated anthocyanins
3. Experimental
3.1. Materials
3.2. Chemicals
3.3. Extraction of anthocyanins
3.4. Qualitative and quantitative analyses of anthocyanins by HPLC-MS
3.5. Statistical analysis
4. Conclusions
Acknowledgements
References and Notes
- Fan, X.; Li, J.M.; Lu, W.J. Malolactic Fermentation (MLF) of Yan73 Grape Wine (In Chinese). Liquor-Making Sci. Tech. 2006, 142, 77–78. [Google Scholar]
- Grimplet, J.; Deluc, L.G.; Tillet, R.L.; Wheatley, M.D.; Schlauch, K.A.; Cramer, G.R.; Cushman, J.C. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics 2007, 187, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Revilla, E. Anthocyanin composition of cabernet sauvignon and tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2003, 51, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Encarna, G.P.; Rocio, G.M.; Alberto, H.J.; Maria, L.R.J.; Ana, O.R.; Adrian, M.C. Studies on the anthocyanin profile of Vitis Vinifera intraspecific hybrids (Monastrell × Cabernet Sauvignon). Eur. Food Res. Technol. 2008, 227, 479–484. [Google Scholar]
- Huang, Z.; Wang, B.; Williams, P.; Pace, R.D. Identification of anthocyanins in muscadine grapes with HPLC-ESI-MS. LWT-Food Sci. Tech. 2009, 42, 819–824. [Google Scholar] [CrossRef]
- Ageorges, A.; Fernandez, L.; Vialet, S.; Merdinoglu, D.; Terrier, N.; Romieu, C. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Sci. 2006, 170, 372–383. [Google Scholar] [CrossRef]
- Balík, J.; Kumšta, M. Evaluation of colour content in grapes originating from South Moravia. Czech J. Food Sci. 2009, 26, 18–24. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; Garcĭa-Romero, E.; Hermosin-Gutiėrrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef] [PubMed]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- Mateus, N.; Pascual-Teresa, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; Freitas, V. Structural diversity of anthocyanin-derived pigments in port wines. Food Chem. 2002, 76, 335–342. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing, a comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Revilla, E.; García-Beneytez, E.; Cabello, F. Anthocyanin fingerprint of clones of Tempranillo grapes and wines made with them. Aust. J. Grape Wine Res. 2009, 15, 70–78. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Fernández-González, M.; Mena, A.; García Romero, E.; Martínez, J. Anthocyanin profile of Spanish Vitis vinifera L. red grape varieties in danger of extinction. Aust. J. Grape Wine Res. 2007, 13, 150–156. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Characterization of anthocyanins in grape juices by ion trap liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2003, 51, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Di Gaspero, G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol 2007, No. 46. [Google Scholar] [CrossRef]
- Bogs, J.; Ebadi, A.; McDavid, D.; Robinson, S.P. Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development. Plant Physiol. 2006, 140, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, H.J.; Park, Y.; Lim, Y.; Ahn, J.H. Characterization of an O-methyltransferase from soybean. Plant Physiol. Biochem. 2006, 44, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Suelves, M.; Puigdomenech, P. Specific mRNA accumulation of a gene coding for an O-methyltransferase in almond (Prunus amygdalus, Batsch) flower tissues. Plant Sci. 1998, 134, 79–88. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Gaspero, G.D.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, R.; Degan, M.; Peterlunger, E.; Gaspero, G.D. Transcriptional regulation of anthocyanins biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Harbone, J.B. The natural distribution in angiosperms of anthocyanins acylated with aliphatic dicarboxylic acids. Phytochemistry 1986, 25, 1887–1894. [Google Scholar] [CrossRef]
- Honda, T.; Saito, N. Recent progress in the chemistry of polyacylated anthocyanins as flower color pigments. Heterocycles 2002, 56, 633–692. [Google Scholar] [CrossRef]
- Nakayama, T.; Suzuki, H.; Nishino, T. Anthocyanin acyltransferases: Specificities, mechanism, phylogenetics, and applications. J. Mol. Cat. B, Enzym. 2003, 23, 117–132. [Google Scholar] [CrossRef]
- Suzuki, H.; Nakayama, T.; Yonekura-Sakakibara, K.; Fukui, Y.; Nakamura, N.; Yamaguchi, M.; Tanaka, Y.; Kusumi, T.; Nishino, T. cDNA cloning, heterologous expressions, and functional characterization of malonyl CoA: Anthocyanidin 3-O-Glucoside-6"-O-Malonyltransferase from dahlia flowers. Plant Physiol. 2002, 130, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. The Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Hopp, W.; Seitz, H.U. The uptake of acylated anthocyanin into isolated vacuoles from a cell suspension culture of Daucus carota. Planta 1987, 170, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Okuno, S.; Yamaguchi, M.; Yamakawa, O. Antimutagenicity of deacylated anthocyanins in purple-fleshed sweetpotato. Biosci. Biotech. Biochem. 2001, 65, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Selection of state of ripeness for harvest and harvesting. In Principles and Practices of Winemaking; Chapman & Hall, Interational Thomson Publishing: New York, NY, USA, 1995; pp. 52–60. [Google Scholar]
- García-Beneytez, E.; Revilla, E.; Cabello, F. Anthocyanin pattern of several red grape cultivars and wines made from them. Eur. Food Res. Technol. 2002, 215, 32–37. [Google Scholar] [CrossRef]
- Wang, H.B.; Edward, J.R.; Shrikhande, A.J. Anthocyanin transformation in Cabernet Sauvignon wine during aging. J. Agric. Food Chem. 2003, 51, 7989–7994. [Google Scholar] [CrossRef] [PubMed]
- Villiers, D.; Vanhonacker, G.; Majek, P.; Sandra, P. Determination of anthocyanins in wine by direct injection liquid chromatography-diode array detection-mass spectrometry and classification of wines using discriminant analysis. J. Chromatogr. A 2004, 1054, 195–204. [Google Scholar] [CrossRef]
- Han, F.L.; Zhang, W.N.; Pan, Q.H.; Zheng, C.R.; Chen, H.Y.; Duan, C.Q. Principal component regression analysis of the relation between cielab Color and monomeric anthocyanins in young cabernet sauvignon wines. Molecules 2008, 13, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- García-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of grape and wine anthocyanins by HPLC-MS. J. Agric. Food Chem. 2003, 51, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Downeya, M.O.; Rochfort, S. Simultaneous separation by reversed-phase high-performance liquid chromatography and mass spectral identification of anthocyanins and flavonols in Shiraz grape skin. J. Chromatogr. A 2008, 1201, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Núñez, V.; Monagas, M.; Gómez-Cordovés, M.C.; Bartolomé, B. Vitis vinifera L. cv. Graciano grapes characterized by its anthocyanin profile. Postharv. Biol. Technol. 2004, 31, 69–79. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
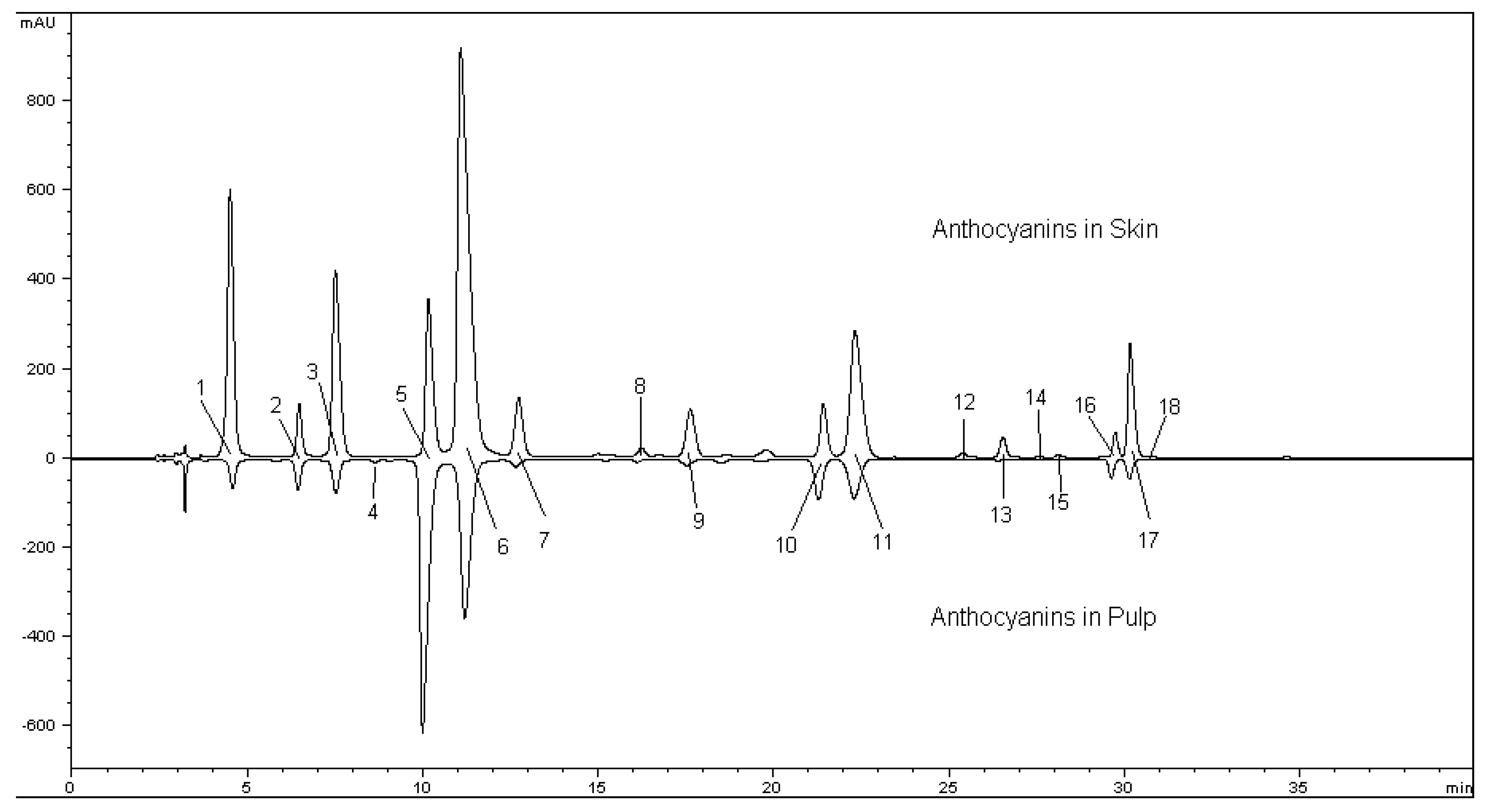
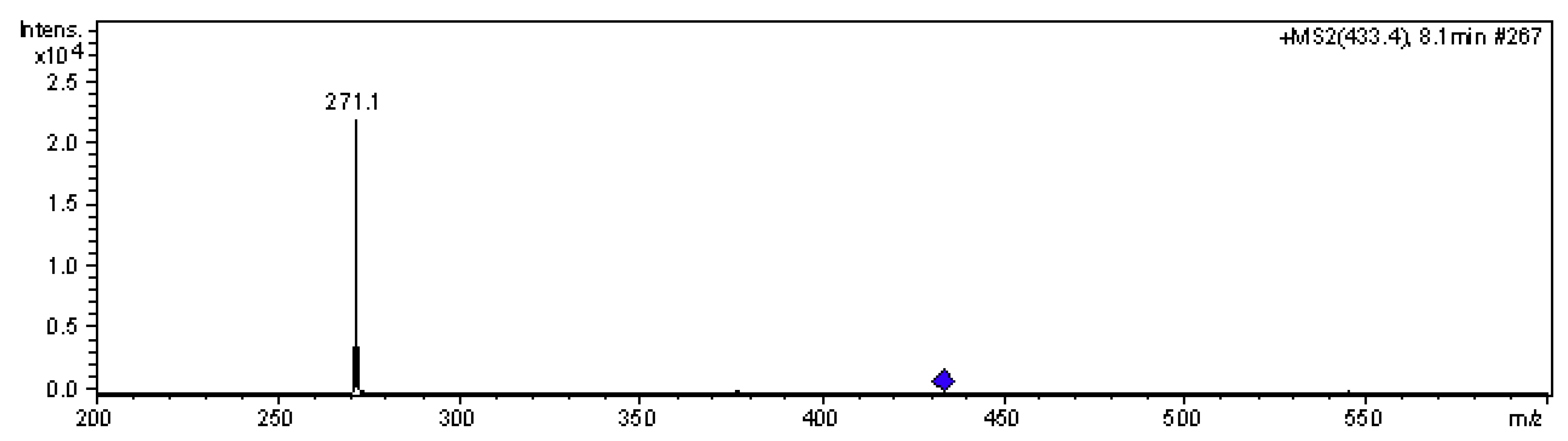
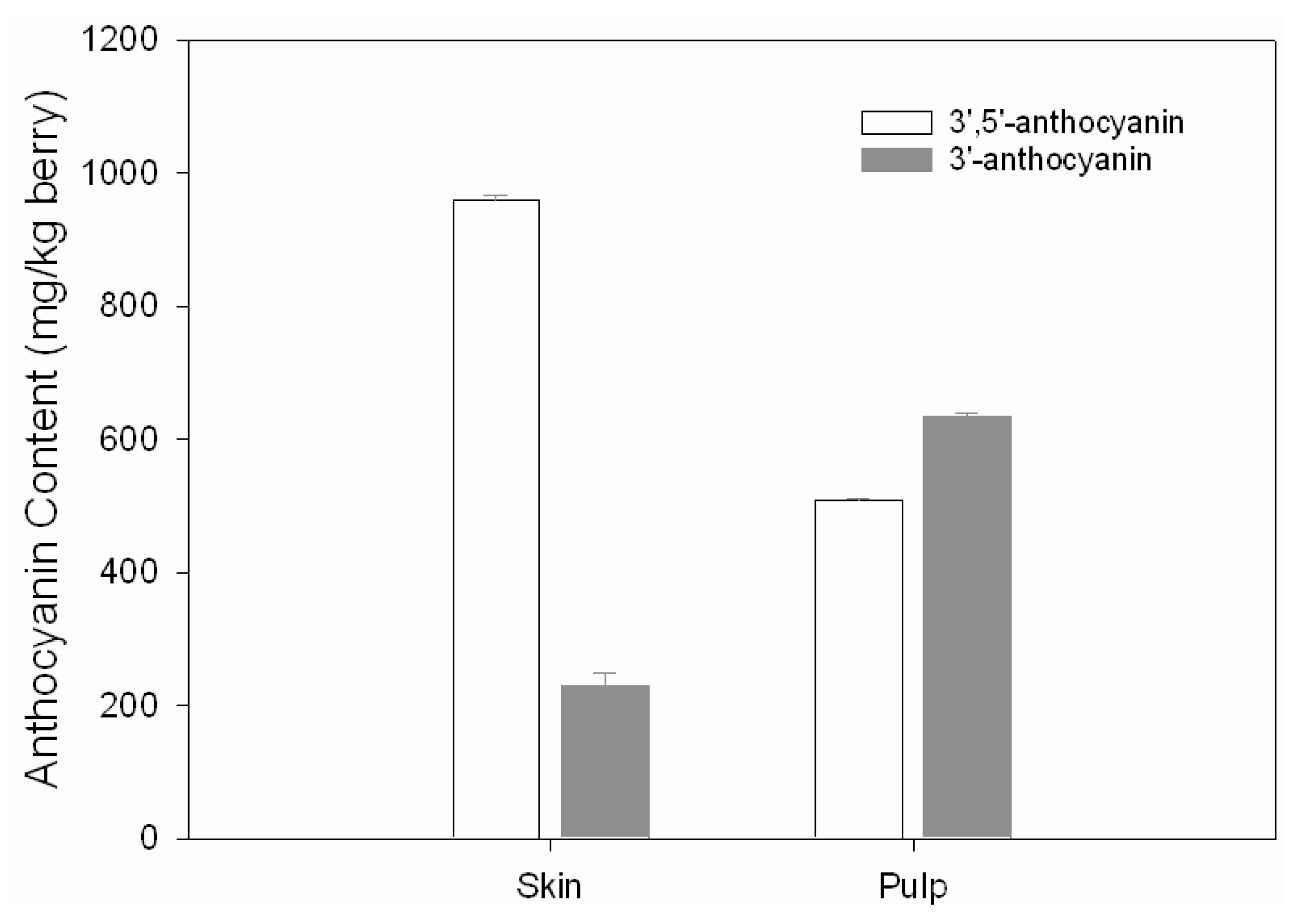

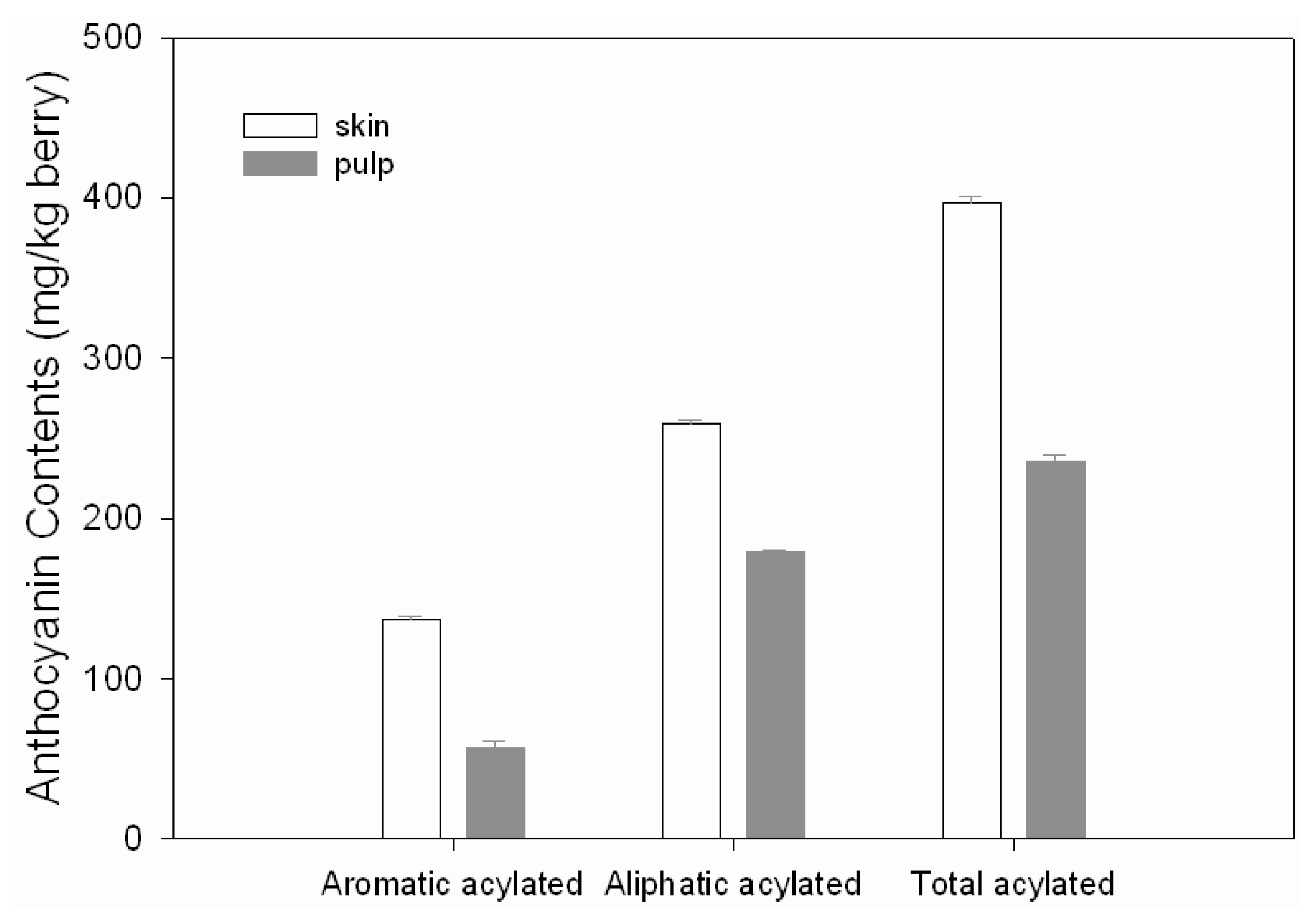
| Peak No | Compound | Antho In Skin /Fresh Berry (mg/kg) | Antho In Pulp /Fresh Berry (mg/kg) | λmax (nm) | M+ & MS2 (m/z) |
|---|---|---|---|---|---|
| 1 | Delphinidin-3-O-glucoside | 56.63 ± 3.06 | 22.54 ± 3.57 | 524 | 465, 303 |
| 2 | Cyanidin-3-O-glucoside | 14.11 ± 0.89 | 22.26 ± 0.94 | 516 | 449, 287 |
| 3 | Petunidin-3-O-glucoside | 56.09 ± 1.57 | 19.61 ± 2.29 | 524 | 479, 317 |
| 4 | Pelargonidin-3-O-glucoside | 2.06 ± 0.62 | 4.79 ± 0.42 | 504 | 433, 271 |
| 5 | Peonidin-3-O-glucoside | 149.91 ± 2.09 | 512.33 ± 11.97 | 518 | 463, 301 |
| 6 | Malvidin-3-O-glucoside | 511.61 ± 3.46 | 323.12 ± 17.32 | 528 | 493, 331 |
| 7 | Delphinidin-3-O-(6-O-acetyl-glucoside) | 19.25 ± 1.86 | 7.3 ± 1.35 | 526 | 507, 303 |
| 8 | Cyanidin-3-O-(6-O-acetyl-glucoside) | 1.38 ± 0.06 | 5.40 ± 0.53 | 522 | 491, 287 |
| 9 | Petunidin-3-O-(6-O-acetyl-glucoside) | 19.92 ± 0.63 | 6.78 ± 1.46 | 522 | 521, 317 |
| 10 | Peonidin-3-O-(6-O-acetyl-glucoside) | 41.93 ± 0.35 | 65.82 ± 2.07 | 522 | 505, 301 |
| 11 | Malvidin-3-O-(6-O-acetyl-glucoside) | 176.54 ± 0.71 | 93.78 ± 5.13 | 528 | 535, 331 |
| 12 | Malvidin-3-O-(6-O-caffeoyl-glucoside) | 2.84 ± 0.05 | 2.51 ± 0.09 | 532 | 655, 331 |
| 13 | Petunidin-3-O-(6-O-coumaryl-glucoside) | 6.58 ± 0.65 | 1.98 ± 0.54 | 530 | 625, 317 |
| 14 | Peonidin-3-O-(cis-6-O-coumaryl-glucoside) | 1.18 ± 0.27 | 1.99 ± 0.17 | 524 | 609, 301 |
| 15 | Malvidin-3-O-( cis-6-O-coumaryl -glucoside) | 4.19 ± 0.58 | 1.73 ± 0.42 | 536 | 639, 331 |
| 16 | Peonidin-3-O-(trans-6-O-coumaryl-glucoside) | 20.64 ± 0.37 | 25.95 ± 1.56 | 522 | 609, 301 |
| 17 | Malvidin-3-O-( trans-6-O-coumaryl-glucoside) | 101.77 ± 0.30 | 22.56 ± 0.64 | 530 | 639, 331 |
| 18 | Malvidin-3-O-(6-O-feuryl-glucoside) | 1.25 ± 0.13 | 1.98 ± 0.30 | 532 | 669, 331 |
| Total anthocyanins | 1,187.88 | 1,142.43 |
© 2010 by the authors;
Share and Cite
He, J.-J.; Liu, Y.-X.; Pan, Q.-H.; Cui, X.-Y.; Duan, C.-Q. Different Anthocyanin Profiles of the Skin and the Pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) Grape Berries. Molecules 2010, 15, 1141-1153. https://doi.org/10.3390/molecules15031141
He J-J, Liu Y-X, Pan Q-H, Cui X-Y, Duan C-Q. Different Anthocyanin Profiles of the Skin and the Pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) Grape Berries. Molecules. 2010; 15(3):1141-1153. https://doi.org/10.3390/molecules15031141
Chicago/Turabian StyleHe, Jian-Jun, Yan-Xia Liu, Qiu-Hong Pan, Xiang-Yun Cui, and Chang-Qing Duan. 2010. "Different Anthocyanin Profiles of the Skin and the Pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) Grape Berries" Molecules 15, no. 3: 1141-1153. https://doi.org/10.3390/molecules15031141





