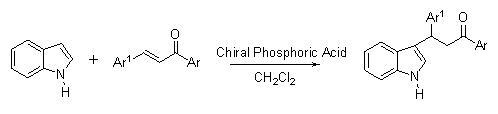
Asymmetric Friedel-Crafts Alkylation of Indole with Chalcones Catalyzed by Chiral Phosphoric Acids
Abstract
:1. Introduction
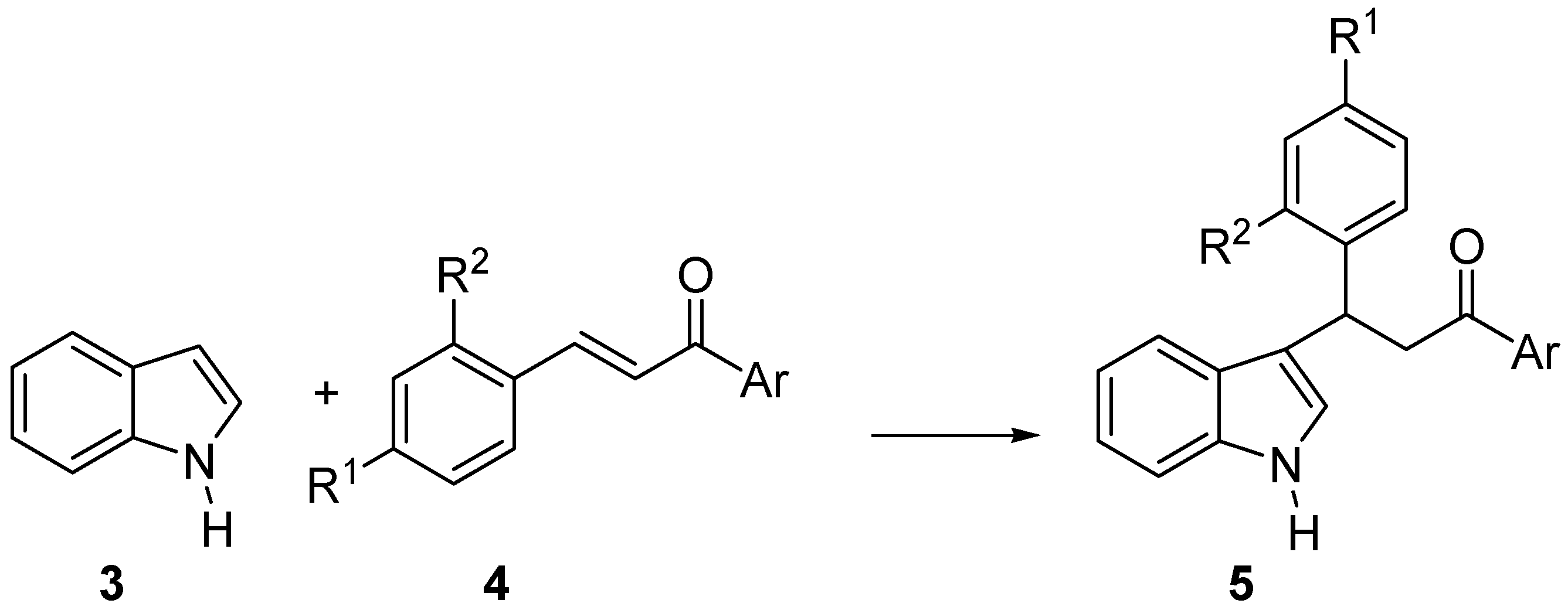
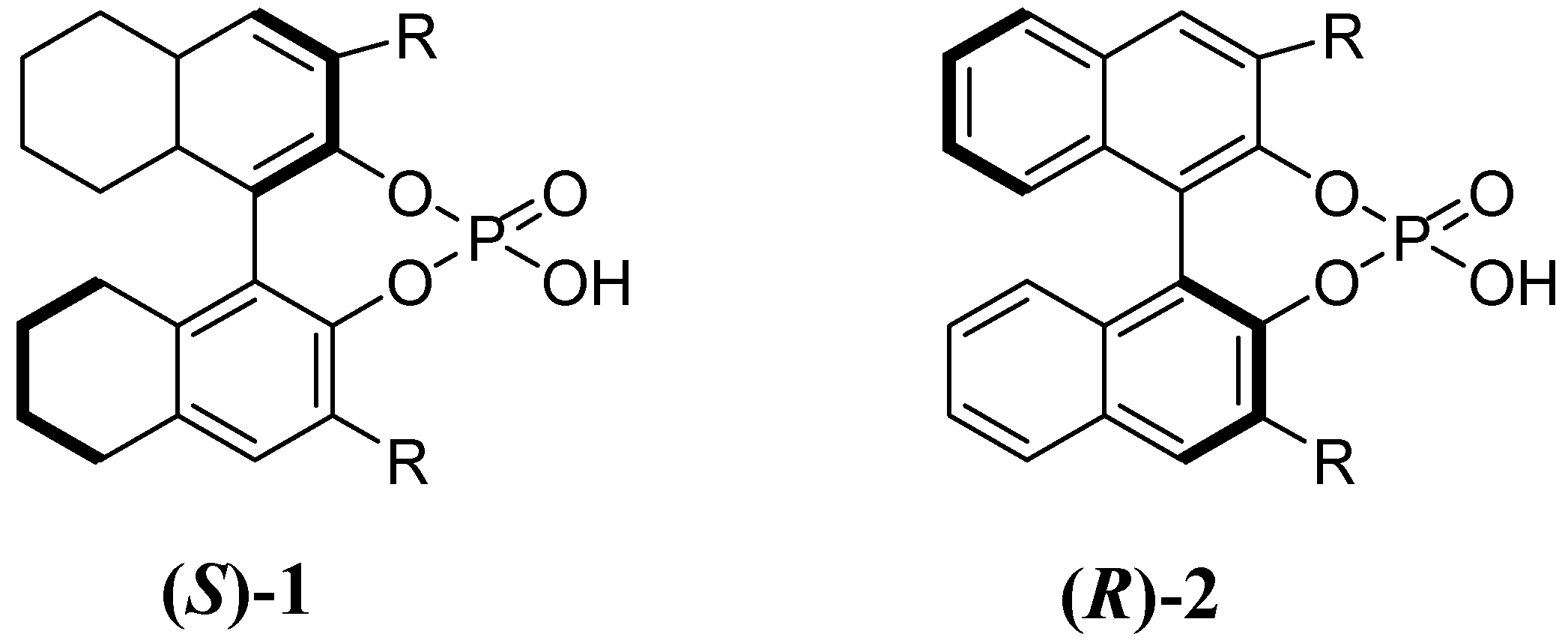
2. Results and Discussion
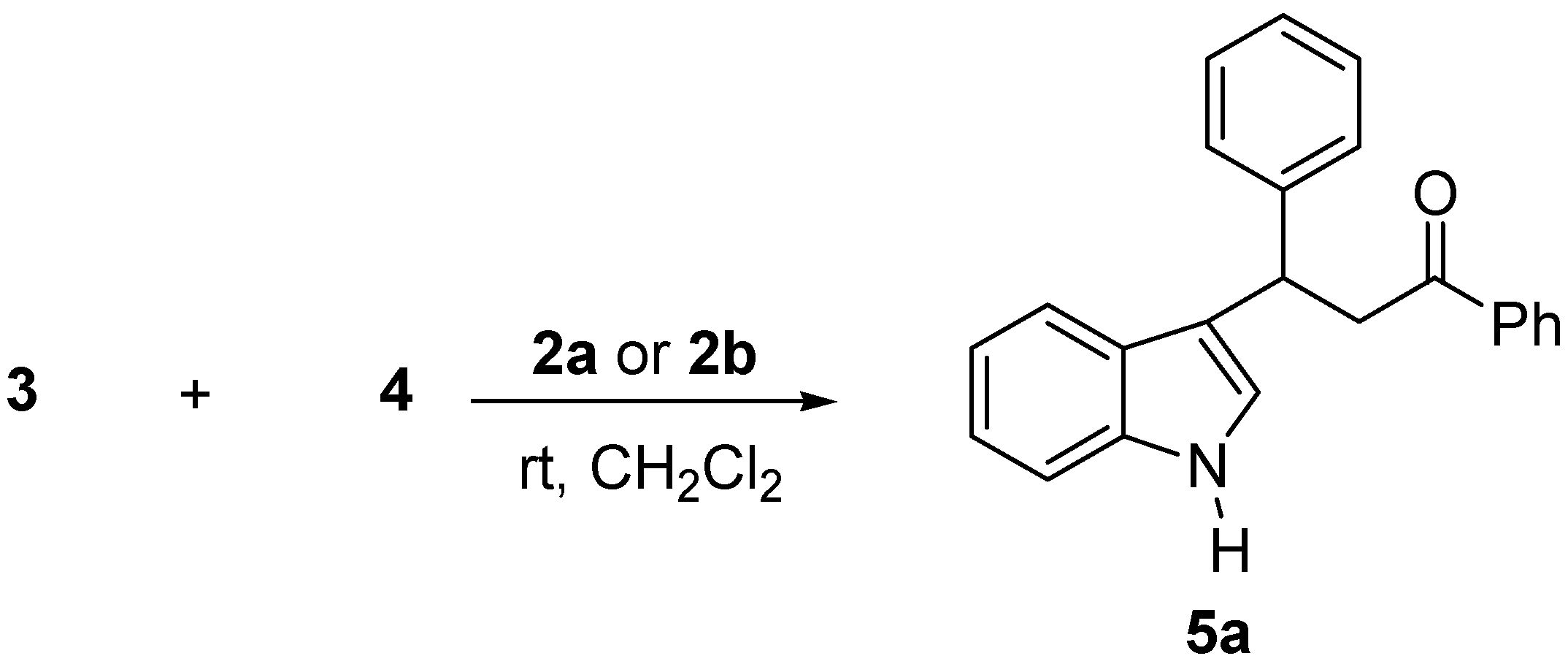
| Entry | Cat. 2 | Reac.Time/h | Yield (%)a | e.e.(%)b |
|---|---|---|---|---|
| 1c,d | 2a (0.05) | 24 | 25 | 40 |
| 2e | 2a (0.05) | 48 | 25 | 49 |
| 3d | 2a (0.1) | 24 | 35 | 33 |
| 4d | 2b (0.1) | 24 | 60 | 31 |
| 5e | 2b (0.1) | 48 | 82 | 52 |
| 6e | 2b (0.05) | 71 | 40 | 48 |
| 7e | 2b (0.02) | 120 | 25 | 46 |

| Entry | Ar | R1 | R2 | Product | Yield(%)a | Ee(%)b,c |
|---|---|---|---|---|---|---|
| 1 | Ph | H | H | 5a | 82(40)d | 52(72) |
| 2 | Ph | H | Cl | 5b | 98 | 48 |
| 3 | Ph | Me | H | 5c | 58(43)d | 52(70) |
| 4 | Ph | OMe | H | 5d | 36 | 41 |
| 5 | Ph | NO2 | H | 5e | 77 | 46 |
| 6 | Ph | Cl | H | 5f | 60(30)d | 52(98) |
| 7 | 4-ClC6H4 | H | H | 5g | 65(33)d | 54(97) |
| 8 | 4-MeC6H4 | H | H | 5h | 44 | 46 |
| 9 | 4-NO2C6H4 | OMe | H | 5i | 73(47)d | 42(51) |
3. Experimental
3.1. General
3.2. Typical experimental procedure
4. Conclusions
Acknowledgements
References and Notes
- Saxton, J.E. Recent progress in the chemistry of the monoterpenoid indole alkaloids. Nat. Prod. Rep. 1997, 14, 559–590. [Google Scholar] [CrossRef]
- Toyota, M.; Ihara, N. Recent progress in the chemistry of non-monoterpenoid indole alkaloids. Nat. Prod. Rep. 1998, 15, 327–340. [Google Scholar] [CrossRef]
- Jorgensen, K.A. Asymmetric Friedel-Crafts reactions: catalytic enantioselective addition of aromatic and heteroaromatic C-H bonds to activated alkenes, carbonyl compounds, and imines. Synthesis 2003, 1117–1125. [Google Scholar] [CrossRef]
- Bandini, M.; Melloni, A.; Umani-Ronchi, A. New catalytic approaches in the stereoselective Friedel-Crafts alkylation reaction. Angew. Chem. Int. Ed. 2004, 43, 550–556. [Google Scholar] [CrossRef]
- Bandini, M.; Fagioli, M.; Melchiorre, P.; Melloni, A.; Umani-Ronchi, A. Catalytic enantioselective conjugate addition of indoles to simple α,β-unsaturated ketones. Tetrahedron Lett. 2003, 44, 5843–5846. [Google Scholar] [CrossRef]
- Blay, G.; Fernandez, I.; Pedro, J.R.; Vila, C. Highly enantioselective Friedel−Crafts alkylations of indoles with simple enones catalyzed by Zirconium(IV)−BINOL complexes. Org. Lett. 2007, 9, 2601–2604. [Google Scholar]
- Jensen, K.B.; Thorhauge, J.; Hazell, R.G.; Jorgensen, K.A. Catalytic asymmetric Friedel-Crafts alkylation of β,γ-unsaturated α-ketoesters: enantioselective addition of aromatic C-H bonds to alkenes. Angew. Chem. Int. Ed. 2001, 40, 160–163. [Google Scholar] [CrossRef]
- Bartoli, G.; Bosco, M.; Carlone, A.; Pesciaioli, F.; Sembri, L.; Melchiorre, P. Organocatalytic asymmetric Friedel−Crafts alkylation of indoles with simple α,β-unsaturated ketones. Org. Lett. 2007, 9, 1403–1405. [Google Scholar] [CrossRef]
- Chen, W.; Du, W.; Yue, L.; Li, R.; Wu, Y.; Ding, L.S.; Chen, Y.C. Organocatalytic enantioselective indole alkylation of α,β-unsaturated ketones. Org. Biomol. Chem. 2007, 5, 816–821. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, L.W.; Li, L.; Yang; Xia, C.G. Enantioselective Michael-Type Friedel-Crafts Reactions of Indoles to Enones Catalyzed by a Chiral Camphor-Based Bronsted. Eur. J. Org. Chem. 2006, 5225–5227. [Google Scholar]
- Thang, H.Y.; Lu, A.D.; Zhou, Z.H.; Zhao, G.F.; He, L.N.; Thang, C.C. Chiral Phosphoric Acid Catalyzed Asymmetric Friedel-Crafts Alkylation of Indoles with Simple α,β-Unsaturated Aromatic Ketones. Eur. J. Org. Chem. 2008, 1406–1410. [Google Scholar]
- For a review on enantioselective C-C bond forming reaction catalyzed by BINOL-derived phosphoric acid: Terada, M. Binaphthol-derived phosphoric acid as a versatile catalyst for enantioselective carbon–carbon bond forming reactions. Chem. Commun. 2008, 35, 4097–4112.
- Sample Availability: Samples of the compounds are available from the authors
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Scettri, A.; Villano, R.; Acocella, M.R. Asymmetric Friedel-Crafts Alkylation of Indole with Chalcones Catalyzed by Chiral Phosphoric Acids. Molecules 2009, 14, 3030-3036. https://doi.org/10.3390/molecules14083030
Scettri A, Villano R, Acocella MR. Asymmetric Friedel-Crafts Alkylation of Indole with Chalcones Catalyzed by Chiral Phosphoric Acids. Molecules. 2009; 14(8):3030-3036. https://doi.org/10.3390/molecules14083030
Chicago/Turabian StyleScettri, Arrigo, Rosaria Villano, and Maria Rosaria Acocella. 2009. "Asymmetric Friedel-Crafts Alkylation of Indole with Chalcones Catalyzed by Chiral Phosphoric Acids" Molecules 14, no. 8: 3030-3036. https://doi.org/10.3390/molecules14083030