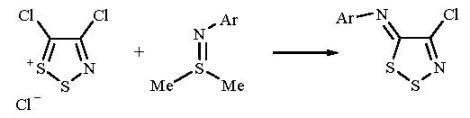
The Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with Sulfimides: A New Synthesis of N-Aryl-1,2,3-dithiazolimines
Abstract
:1. Introduction
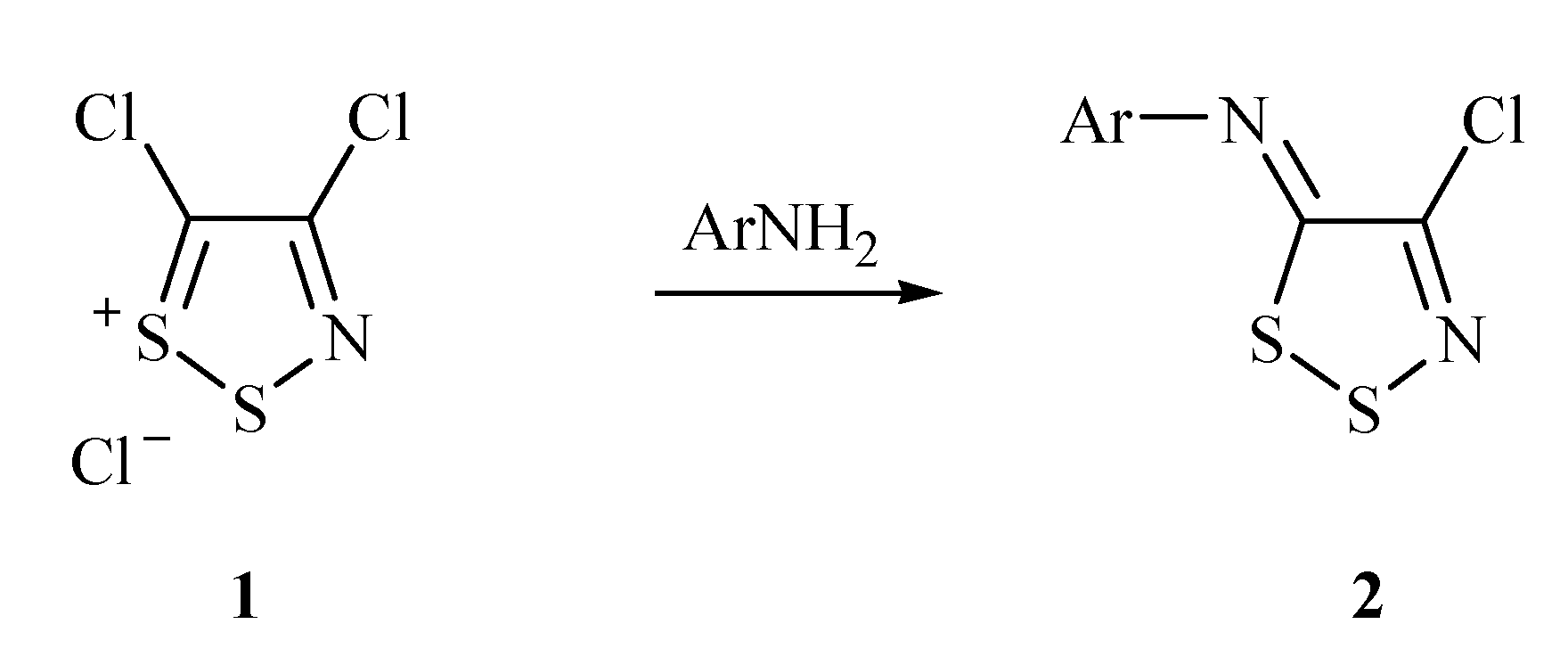
2. Results and Discussion
 |
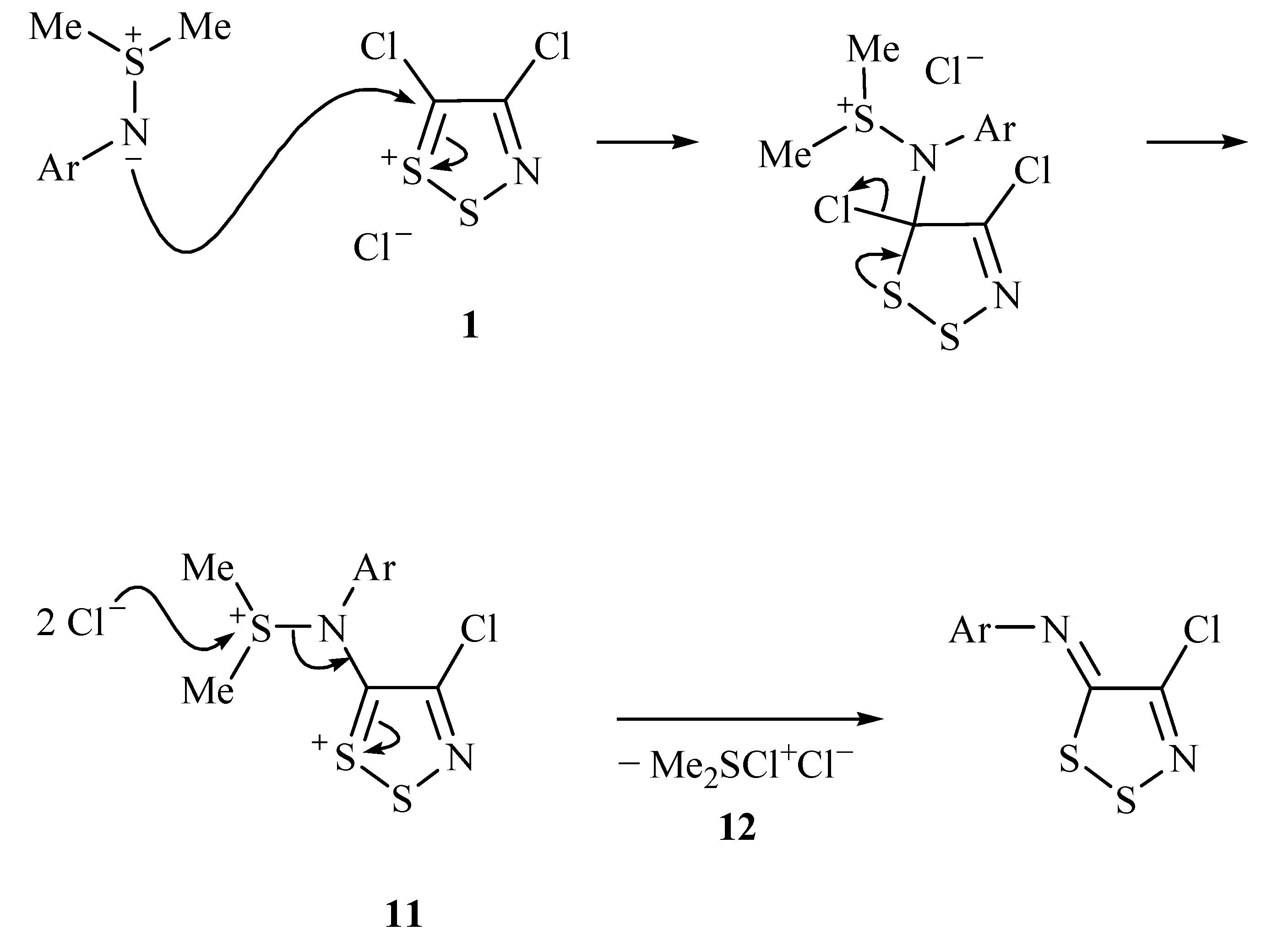
3. Conclusions
4. Experimental
4.1. General
4.2. Reactions of Appel salt 1 with sulfimides: Typical procedure (see Table 1)
4.3. Reactions of Appel salt 1 with anilines: Typical procedure [21] (see Table 1)
Acknowledgements
References and Notes
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, S.; Thiéry, V.; Rakitin, O.A. One-pot Synthesis of 5-Phenylimino, 5-Thieno or 5-Oxo-1,2,3-dithiazoles and Evaluation of their Antimicrobial and Antitumor Activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar] [CrossRef]
- Cottenceau, G.; Besson, T.; Gautier, V.; Rees, C.W.; Pons, A.M. Antibacterial Evaluation of Novel N-Arylimino-1,2,3-dithiazoles and N-Arylcyanothioformamides. Bioorg. Med. Chem. Lett. 1996, 6, 529–532. [Google Scholar] [CrossRef]
- Thiery, V.; Rees, C.W.; Besson, T.; Cottenceau, G.; Pons, A.M. Antimicrobial Activity of Novel N-Quinolinyl and N-Naphthylimino-1,2,3-dithiazoles. Eur. J. Med. Chem. 1998, 33, 149–153. [Google Scholar] [CrossRef]
- Joseph, R.W.; Antes, D.L.; Osei-Gyimah, P. Antimicrobial Compounds with Quick Speed of Kill. US Pat. 5688744.
- Moore, J.E. Certain 4-Halo-5-aryl-1,2,3-dithiazole Compounds and their Preparation. US Pat. 4059590, 1977. [Google Scholar]
- Appel, R.; Janssen, H.; Haller, I.; Plempel, M. 1,2,3-Dithiazolderivate, Verfahren zu ihrer Herstellung Sowie ihre Verwendung als Arzneimittel. DE Pat. 2848221.
- Besson, T.; Rees, C.W.; Cottenceau, G.; Pons, A.M. Antimicrobial Evaluation of 3,1-Benzoxazin-4-ones, 3,1-Benzothiazin-4-ones, 4-Alkoxyquinazolin-2-carbonitriles and N-Arylimino-1,2,3-dithiazoles. Bioorg. Med. Chem. Lett. 1996, 6, 2343–2348. [Google Scholar] [CrossRef]
- Mayer, R.; Foerster, E.; Matauschek, B. Verfahren zur Herstellung von Aromatisch oder Heteroaromatisch Substituierten Cyanthioformamiden. DD Pat. 212387, 1984. [Google Scholar]
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and Properties of 1,2,3-Dithiazoles, Russ. Chem. Rev. 2008, 77, 521–546. [Google Scholar]
- van der Plas, H.C. Chapter II SN(ANRORC) Reactions in Azines, Containing an “Outside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 9–86. [Google Scholar]
- van der Plas, H.C. Chapter III SN(ANRORC) Reactions in Azaheterocycles Containing an “Inside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 87–151. [Google Scholar]
- Rees, C.W. Polysulfur-nitrogen Heterocyclic Chemistry. J. Heterocycl. Chem. 1992, 29, 639–651. [Google Scholar] [CrossRef]
- Besson, T.; Dozias, M.J.; Guillard, J.; Rees, C.W. New Route to 2-Cyano-benzothiazoles via N-Arylimino-1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1998, 3925–3926. [Google Scholar]
- Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Direct Synthesis of 2-Cyano-benzimidazoles and the Generation of S2. Tetrahedron Lett. 1996, 37, 4589–4592. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Michaelidou, S.S. 1,2,3-Dithiazole Chemistry in Heterocyclic Synthesis. Arkivoc 2006, 7, 207–223. [Google Scholar]
- Besson, T.; Guillaumet, G.; Lamazzi, C.; Rees, C.W. Synthesis of 3,1-Benzoxazines, 3,1-Benzothiazines and 3,1-Benzoxazepines via N-Arylimino-1,2,3-dithiazoles. Synlett 1997, 704–706. [Google Scholar]
- Rakitin, O.A. Comprehensive Heterocyclic Chemistry, 3rd ed.; Zhdankin, V.V., Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; vol. 6, ch. 6.01; p. 1. [Google Scholar]
- Kim, K. Synthesis and Reactions of 1,2,3-Dithiazoles. J. Sulfur Chem. 1998, 21, 147–207. [Google Scholar]
- Kim, K. Recent Advances in 1,2,3-Dithiazole Chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1997, 120, 229–244. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- English, R.F.; Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Conversion of Imino-1,2,3-dithiazoles into 2-Cyanobenzothiazoles, Cyanoimidoyl Chlorides and Diatomic Sulfur. J. Chem. Soc. Perkin Trans. 1 1997, 201–206. [Google Scholar]
- English, R.F. Thesis, University of London, 1989.
- Cuadro, A.M.; Alvarez-Buila, J. 4,5-Dichloro-1,2,3-dithiazolium Chloride (Appel's Salt): Reactions with N-Nucleophiles. Tetrahedron 1994, 50, 10037–10046. [Google Scholar] [CrossRef]
- Gilchrist, T.L.; Harris, C.J.; Hawkins, D.G.; Moody, C.J.; Rees, C.W. Synthesis of 1H-1,2,4-Triazole 2-Oxides and Annelated Derivatives. J. Chem. Soc. Perkin Trans. 1 1976, 2166–2170. [Google Scholar]
- Alcaide, B.; Casarrubios, L.; Domhguez, G.; Sierra, M.A. Reaction of Chromium (Fischer) Carbenes and Sulfilimines. J. Org. Chem. 1993, 58, 3886–3894. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ku, T.; Dawson, A.D.; Swern, D. Iminosulfuranes. XV. Dimethyl Sulfoxide-trifluoroacetic Anhydride. New and Efficient Reagent for the Preparation of Iminosulfuranes. J. Org. Chem. 1975, 40, 2758–2764. [Google Scholar] [CrossRef]
- Claus, P.K.; Rieder, W.; Hofbauer, P.; Vilsmaier, E. N-Aryl Sulfimides. Tetrahedron 1975, 31, 505–510. [Google Scholar] [CrossRef]
- Warthmann, W.; Schmidt, A. Reaktionsprodukte aus Chlorsulfonium-Salzen und Alkoholen bzw. Wasser und deren IR-Spektren. Chem. Ber. 1975, 108, 520–527. [Google Scholar] [CrossRef]
- Chasar, D.W.; Pratt, T.M.; Shockcor, J.P. The Reaction of Anhydrous HCl/Chloroform with Diaryl Sulfoxides. Phosphorus Sulfur Silicon Relat. Elem. 1980, 8, 183–186. [Google Scholar]
- Madesclaire, M. Reduction of Sulfoxides to Thioethers. Tetrahedron 1988, 44, 6537–6580. [Google Scholar] [CrossRef]
- Claus, P.; Vycudilik, W. Methylthiomethylierung von Aromatischen Aminen: N-Aryl-S,S-dimethylsulfimide als Zwischenstufen. Tetrahedron Lett. 1968, 9, 3607–3610. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the corresponding author.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. The Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with Sulfimides: A New Synthesis of N-Aryl-1,2,3-dithiazolimines. Molecules 2009, 14, 2356-2362. https://doi.org/10.3390/molecules14072356
Kalogirou AS, Koutentis PA. The Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with Sulfimides: A New Synthesis of N-Aryl-1,2,3-dithiazolimines. Molecules. 2009; 14(7):2356-2362. https://doi.org/10.3390/molecules14072356
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2009. "The Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with Sulfimides: A New Synthesis of N-Aryl-1,2,3-dithiazolimines" Molecules 14, no. 7: 2356-2362. https://doi.org/10.3390/molecules14072356