Quantitative Structure ‒ Antiprotozoal Activity Relationships of Sesquiterpene Lactones
Abstract
:1. Introduction
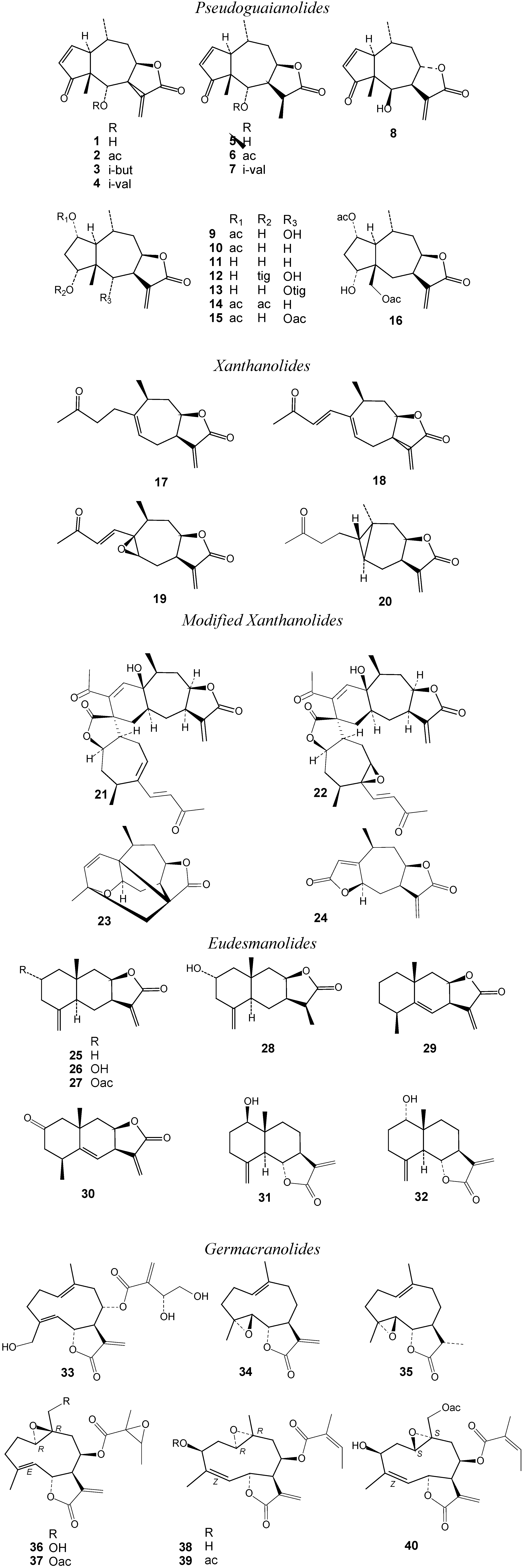
2. Results and Discussion
2.1. Biological Activity Data and Activity-Activity Relationships
| Compound | T. brucei rhodes. (Tbr) | T. cruzi(Tcr) | L. donovani(Ldon) | P. falciparum(Pfc) | Cytotoxicity (L6) |
|---|---|---|---|---|---|
| 1 | 0.052 | 0.695 | n.t. | n.t. | 0.992 |
| 2 | 0.063 | 0.538 | 0.446 | 0.329 | 0.809 |
| 3 | 0.105 | 1.568 | 0.837 | 0.700 | 1.030 |
| 4 | 0.116 | 2.478 | 0.871 | 0.822 | 1.298 |
| 5 | 0.686 | 21.477 | 3.848 | 3.047 | 8.902 |
| 6 | 1.415 | 6.928 | >4* | n.t. | 3.056 |
| 7 | 0.911 | 3.534 | 1.476 | 1.603 | 4.583 |
| 8 | 0.319 | 1.870 | >4* | n.t. | 2.443 |
| 9 | 9.275 | 45.833 | n.t. | n.t. | 12.284 |
| 10 | 6.705 | 20.422 | 12.240 | 6.461 | 4.392 |
| 11 | 18.346 | 52.744 | 20.602 | 12.143 | 31.917 |
| 12 | 10.951 | 49.176 | 17.967 | 10.604 | 16.676 |
| 13 | 19.242 | 60.165 | 14.505 | 27.473 | 31.758 |
| 14 | 1.174 | 14.657 | 4.257 | 8.943 | 8.429 |
| 15 | 3.961 | 16.311 | 8.675 | 5.984 | 7.281 |
| 16 | 13.607 | 44.891 | 11.831 | 10.656 | 21.557 |
| 17 | 62.081 | >120* | 23.831 | >20* | 178.790 |
| 18 | 2.919 | 26.220 | 17.642 | 7.825 | 30.203 |
| 19 | 0.330 | 11.260 | 0.599 | 6.511 | 22.137 |
| 20 | 15.887 | 38.992 | 14.597 | 12.702 | 19.839 |
| 21 | 0.638 | 16.083 | 22.047 | 4.961 | 9.429 |
| 22 | 1.288 | 95.420 | 26.908 | 6.527 | 13.282 |
| 23 | 162.200 | >120* | 15.400 | >20* | >365* |
| 24 | 8.205 | >120* | 27.051 | >20* | 427.350 |
| 25 | 23.621 | 22.263 | >14* | n.t. | 4.483 |
| 26 | 7.802 | 24.778 | >4* | n.t. | 0.948 |
| 27 | 10.724 | 24.345 | 11.759 | 9.207 | 8.914 |
| 28 | 341.480 | >120* | >120* | >20* | 187.120 |
| 29 | 2.625 | 8.297 | 3.030 | 6.401 | 5.991 |
| 30 | 1.602 | 17.256 | 8.699 | 10.691 | 10.183 |
| 31 | 17.806 | 28.911 | 6.008 | 12.056 | 33.185 |
| 32 | 1.107 | 26.210 | 7.339 | 13.629 | 25.565 |
| 33 | 1.303 | 16.799 | 15.212 | 6.323 | 10.212 |
| 34 | 0.388 | 10.665 | 3.556 | 11.895 | 7.238 |
| 35 | 49.000 | >120* | 35.560 | >20* | 132.600 |
| 36 | 14.283 | 25.582 | 2.726 | 9.444 | 13.624 |
| 37 | 0.478 | 15.786 | 5.190 | 6.333 | 7.750 |
| 38 | 0.942 | 16.492 | 18.204 | 8.812 | 12.970 |
| 39 | 0.804 | 17.574 | 30.074 | 8.069 | 10.099 |
| 40 | 0.698 | 11.833 | 17.095 | 6.643 | 6.667 |
| pos. control | 0.008a | 2.000b | 0.270c | 0.248d | 0.012e |
| T. b. rhod. | T.cruzi | L. donov. | P. falcip. | L6 cytotox. | |
|---|---|---|---|---|---|
| T. b. rhod. | 1.000 | ||||
| T.cruzi | 0.851 | 1.000 | |||
| L. donov. | 0.704 | 0.823 | 1.000 | ||
| P. falcip. | 0.808 | 0.912 | 0.753 | 1.000 | |
| L6 cytotox. | 0.698 | 0.857 | 0.697 | 0.905 | 1.000 |
2.2. Structure-activity relationships, QSAR
| Model | n var.a | n comp.b | n PCsc | R2 | Q2 | ||
|---|---|---|---|---|---|---|---|
| Tbr | L6 | Tbr | L6 | ||||
| 1 | 44 | 40 | 7 | 0.83 | 0.91 | 0.52 | 0.41 |
| 2 | 44 | 39* | 4 | 0.81 | 0.82 | 0.66 | 0.61 |
| 3 | 20 | 39* | 3 | 0.80 | 0.81 | 0.71 | 0.71 |

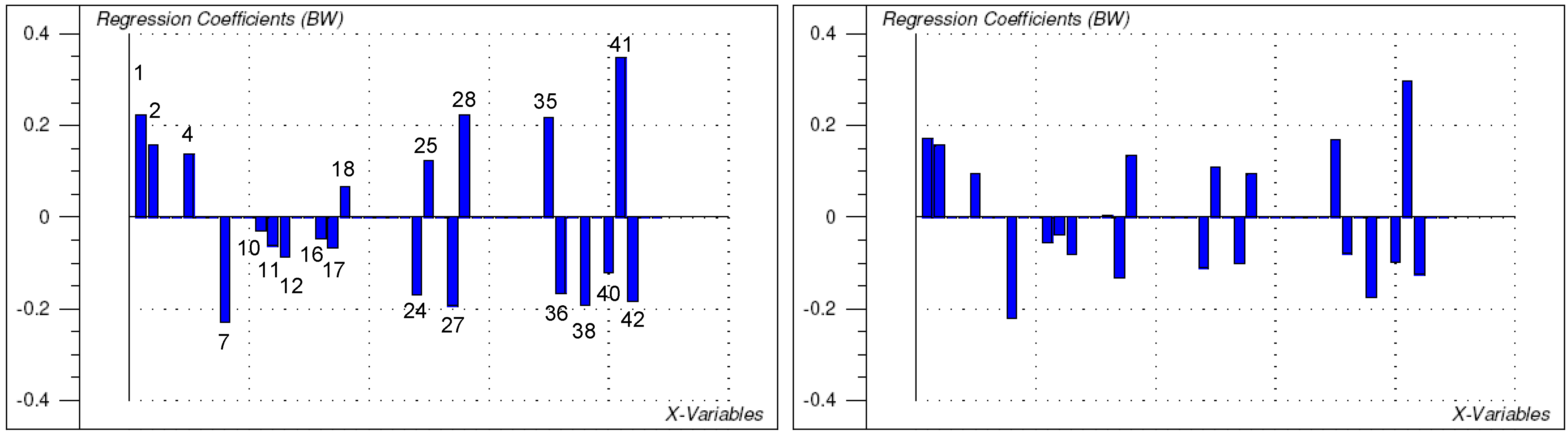
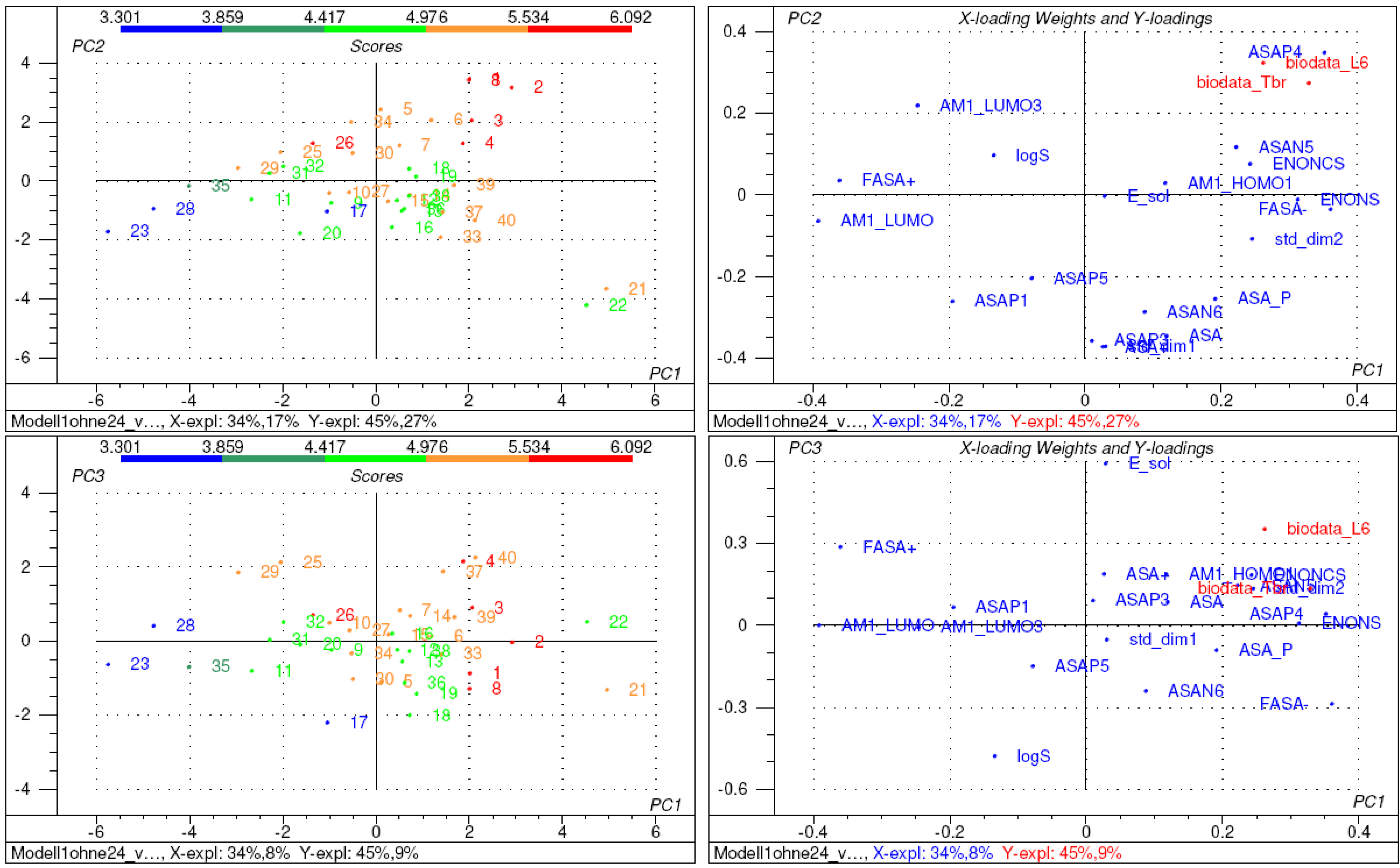
3. Conclusions
4. Experimental
4.1. Test compounds
4.2. In vitro assays and IC50 determination
4.3. Computational Methods
| 1 | ENONCS | accessible surface area of beta carbons in α,β-unsaturated carbonyl structures |
| 2 | ENONS | accessible surface area of α,β-unsaturated carbonyl structures |
| 2 | AM1_HOMO | Eigenvalue of highest occupied molecular orbital (MOE/MOPAC/AM1) |
| 4 | AM1_HOMO1 | Eigenvalue of second highest occupied molecular orbital (MOE/MOPAC/AM1) |
| 5 | AM1_HOMO2 | Eigenvalue of third highest occupied molecular orbital (MOE/MOPAC/AM1) |
| 6 | AM1_HOMO3 | Eigenvalue of fourth highest occupied molecular orbital (MOE/MOPAC/AM1) |
| 7 | AM1_LUMO | Eigenvalue of lowest unoccupied molecular orbital (MOE/MOPAC/AM1) |
| 8 | AM1_LUMO1 | Eigenvalue of second lowest unoccupied molecular orbital (MOE/MOPAC/AM1) |
| 9 | AM1_LUMO2 | Eigenvalue of third lowest unoccupied molecular orbital (MOE/MOPAC/AM1) |
| 10 | AM1_LUMO3 | Eigenvalue of fourth lowest unoccupied molecular orbital (MOE/MOPAC/AM1) |
| 11 | AM1_dipole | Dipole moment calculated with MOE/MOPAC/AM1 |
| 12 | ASA | solvent accessible molecular surface area [11] |
| 13 | ASA+ | solvent accessible molecular surface area due to atoms with positive parial charge [11] |
| 14 | ASA- | solvent accessible molecular surface area due to atoms with negative parial charge [11] |
| 15 | ASA_H | solvent accessible molecular surface area due to atoms with hydrophobic properties [11] |
| 16 | ASA_P | solvent accessible molecular surface area due to atoms with polar properties [11] |
| 17 | FASA+ | fractional solvent accessible molecular surface area calculated as ASA+/ASA [11] |
| 18 | FASA- | fractional solvent accessible molecular surface area calculated as ASA-/ASA [11] |
| 19 | FASA_H | fractional solvent accessible molecular surface area calculated as ASA_H/ASA [11] |
| 20 | FASA_P | fractional solvent accessible molecular surface area calculated as ASA_P/ASA [11] |
| 21 | FCASA+ | Positive charge weighted surface area, ASA+ * maximum positive partial charge [11] |
| 22 | FCASA- | Negative charge weighted surface area, ASA- * maximum negative partial charge [11] |
| 23 | glob | globularity [11] |
| 24 | std_dim1 | largest standardized dimension [11] |
| 25 | std_dim2 | second largest standardized dimension [11] |
| 26 | std_dim3 | third largest standardized dimension [11] |
| 27 | logS | log of calculated water solubility [11] |
| 28 | E_sol | solvation Energy [11] |
| 29 | logP(o/w) | log of calculated octanol/water partition coefficient [11] |
| 30 | SlogP | log of calculated octanol/water partition coefficient [11] |
| 31 | ASAN1 | fractional accessible surface area due to atoms in partial charge interval 0 to -0.05 e [7] |
| 32 | ASAN2 | fractional accessible surface area due to atoms in partial charge interval -0.05 to -0.1 e [7] |
| 33 | ASAN3 | fractional accessible surface area due to atoms in partial charge interval -0.1 to -0.15 e [7] |
| 34 | ASAN4 | fractional accessible surface area due to atoms in partial charge interval -0.15 to -0.2 e [7] |
| 35 | ASAN5 | fractional accessible surface area due to atoms in partial charge interval -0.2 to -0.25 e [7] |
| 36 | ASAN6 | fractional accessible surface area due to atoms in partial charge interval -0.25 to -0.3 e [7] |
| 37 | ASAN7 | fractional accessible surface area due to atoms in partial charge interval <-0.30 e [7] |
| 38 | ASAP1 | fractional accessible surface area due to atoms in partial charge interval 0 to 0.05 e [7] |
| 39 | ASAP2 | fractional accessible surface area due to atoms in partial charge interval 0.05 to 0.1 e [7] |
| 40 | ASAP3 | fractional accessible surface area due to atoms in partial charge interval 0.1 to 0.15 e [7] |
| 41 | ASAP4 | fractional accessible surface area due to atoms in partial charge interval 0.15 to 0.2 e [7] |
| 42 | ASAP5 | fractional accessible surface area due to atoms in partial charge interval 0.2 to 0.25 e [7] |
| 43 | ASAP6 | fractional accessible surface area due to atoms in partial charge interval 0.25 to 0.3 e [7] |
| 44 | ASAP7 | fractional accessible surface area due to atoms in partial charge interval >0.3 e [7] |
Acknowledgements
References and Notes
- World Health Organization, 2004. Global burden of disease report update, 2004. Available: http://www.who.int/ healthinfo/global_burden_disease/en/.
- World Health Organization, 2007. Available: http://www.who.int/features/factfiles/neglected_tropical_diseases/en/index.html.
- Hoet, S.; Opperdoes, F.; Brun, R.; Quetin-Leclercq, J. Natural products active against African trypanosomes: a step towards new drugs. Nat. Prod. Rep. 2004, 21, 353–364. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Willuhn, G.; Brun, R.; Khalid, S.A. Antitrypanosomal activity of Helenalin and some related Sesquiterpene Lactones. Planta Med. 2002, 68, 750–751. [Google Scholar] [CrossRef]
- Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R.; Abdallah, W. E.; Schmidt, T.J. The antiprotozoal activity of sixteen Asteraceae species native to Sudan and bioactivity-guided isolation of xanthanolides from Xanthium brasilicum Vell. Planta Med. 2009, in press. [Google Scholar] [CrossRef]
- Schmidt, T.J. Quantitative structure-cytotoxicity relationships within a series of helenanolide type sesquiterpene lactones. (Helenanolide Type Sesquiterpene Lactones, IV.). Pharm. Pharmacol. Lett. 1999, 9, 9–13. [Google Scholar]
- Schmidt, T.J.; Heilmann, J. Quantitative Structure-Cytotoxicity Relationships of Sesquiterpene Lactones derived from Partial Charge (Q)-based Fractional Accessible Surface Area Descriptors (Q_frASAs). Quant. Struct.-Act. Relat. (QSAR) 2002, 21, 276–287. [Google Scholar] [CrossRef]
- Schmidt, T.J. Toxic Activities of Sesquiterpene Lactones – Structural and Biochemical Aspects. Current Org. Chem. 1999, 3, 577–605. [Google Scholar]
- Schmidt, T.J. Structure-Activity Relationships of Sesquiterpene Lactones. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Vol. 33, pp. 309–392. [Google Scholar]
- Beekman, A.C.; Woerdenbag, H.J.; van Uden, W.; Pras, N.; Konings, A.W.T.; Wikström, H.; Schmidt, T.J. Structure-Cytotoxicity Relationships of some Helenanolide-Type Sesquiterpene Lactones. J. Nat. Prod. 1997, 60, 252–257. [Google Scholar] [CrossRef]
- MOE - Molecular Operating Environment v. 2008.10, available from Chemical Computing Group, Montreal, Canada: http://www.chemcomp.com/; For details on molecular descriptors see: http://www.chemcomp.com/journal/descr.htm
- The Unscrambler, v. 9.2. available from Chemical Computing Group, Montreal, Canada: http://www.camo.no.
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley and Sons: London, U.K., 1976. [Google Scholar]
- De Carvalho, P.B.; Ramos, D.C.C.; Cotrim, P.C.; Ferreira, E.I. Synthesis and in vitro evaluation of potential anti-leishmanial targeted drugs of pyrimethamine. J. Pharm. Sci. 2003, 92, 2109–2116. [Google Scholar] [CrossRef]
- Chung, M.-C.; Carvalho Guido, R.V.; Martinelli, T.F.; Ferreira Goncalves, M.; Carneiro Polli, M.; Alves Botelho, K.C.; Aparecida Varanda, E.; Colli, W.; Mirando, M.T.M.; Ferriera, E.I. Synthesis and in vitro evaluation of potential antichagasic hydroxymethylnitrofurazone (NFOH-121): a new nitrofurazone prodrug. Bioorg. Med. Chem. 2003, 11, 4779–4783. [Google Scholar] [CrossRef]
- Willuhn, G.; Merfort, I.; Paßreiter, C.M.; Schmidt, T.J. Chemistry and Systematics of the Genus Arnica. In Advances in Compositae Systematics; Hind, D.J.N., Jeffrey, C., Pope, G.V., Eds.; Royal Botanic Gardens: Kew, UK, 1994; pp. 167–195. [Google Scholar]
- Budesinsky, M.; Saman, S. Carbon-13 NMR Spectra of Sesquiterpene Lactones. Ann. Rep. NMR Spect. 1995, 30, 231–475. [Google Scholar] [CrossRef]
- Bohlmann, F.; Mahanta, P.K.; Jakupovic, J.; Rastogi, R.C.; Natu, A.A. New Sesquiterpene Lactones from Inula species. Phytochemistry 1978, 17, 1165–1172. [Google Scholar] [CrossRef]
- Matile, H.; Pink, J.R.L. Plasmodium falciparum malaria parasite cultures and their use in immunology. In Immunological Methods; Lefkovits, I., Pernis, B., Eds.; Academic Press: San Diego, USA, 1990; pp. 221–234. [Google Scholar]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivitiy to African Trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar]
- Cunningham, I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J. Protozool. 1977, 23, 325–329. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the corresponding author.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative Structure ‒ Antiprotozoal Activity Relationships of Sesquiterpene Lactones. Molecules 2009, 14, 2062-2076. https://doi.org/10.3390/molecules14062062
Schmidt TJ, Nour AMM, Khalid SA, Kaiser M, Brun R. Quantitative Structure ‒ Antiprotozoal Activity Relationships of Sesquiterpene Lactones. Molecules. 2009; 14(6):2062-2076. https://doi.org/10.3390/molecules14062062
Chicago/Turabian StyleSchmidt, Thomas J., Amal M. M. Nour, Sami A. Khalid, Marcel Kaiser, and Reto Brun. 2009. "Quantitative Structure ‒ Antiprotozoal Activity Relationships of Sesquiterpene Lactones" Molecules 14, no. 6: 2062-2076. https://doi.org/10.3390/molecules14062062






