Synthesis and Analysis of Resorcinol-Acetone Copolymer
Abstract
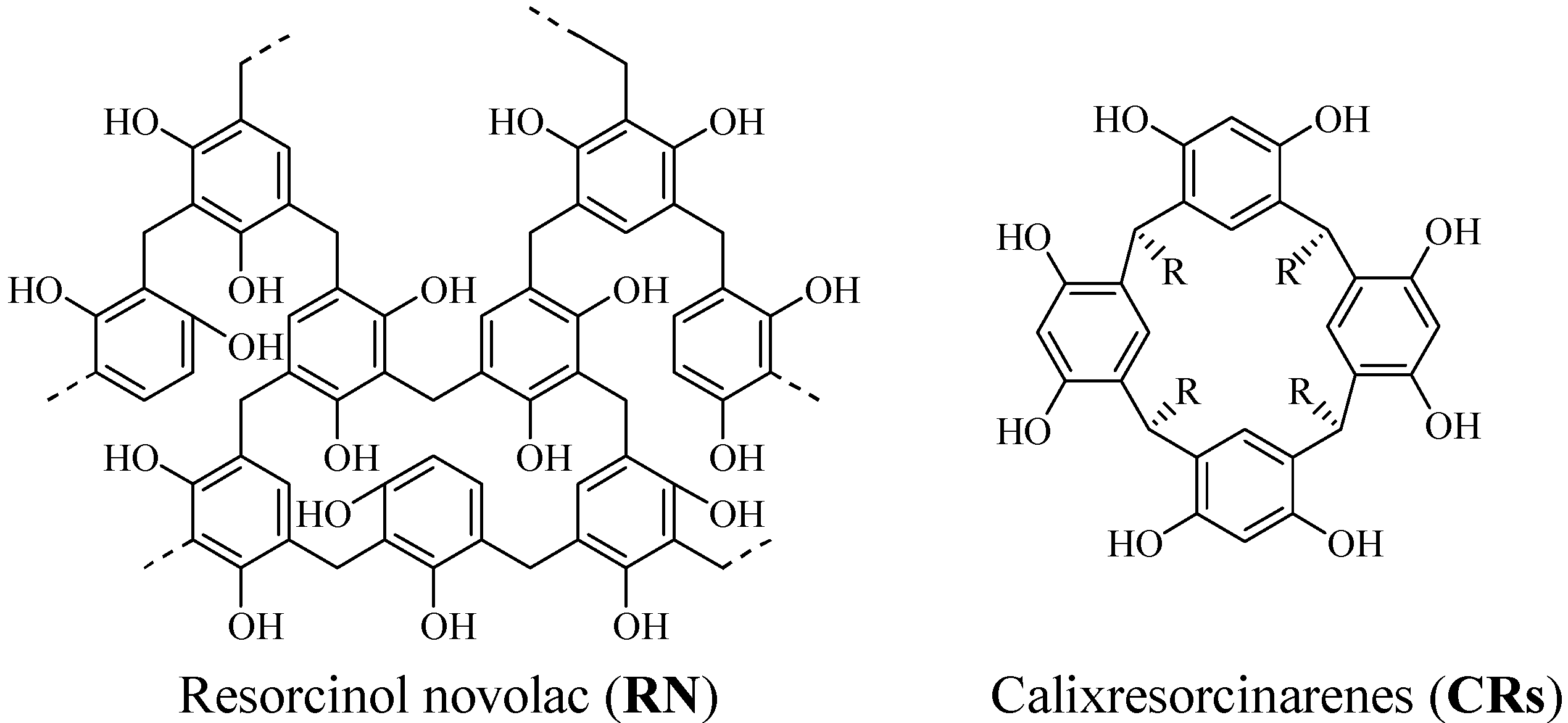
:Introduction
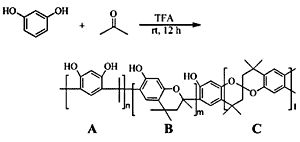
Results and Discussion
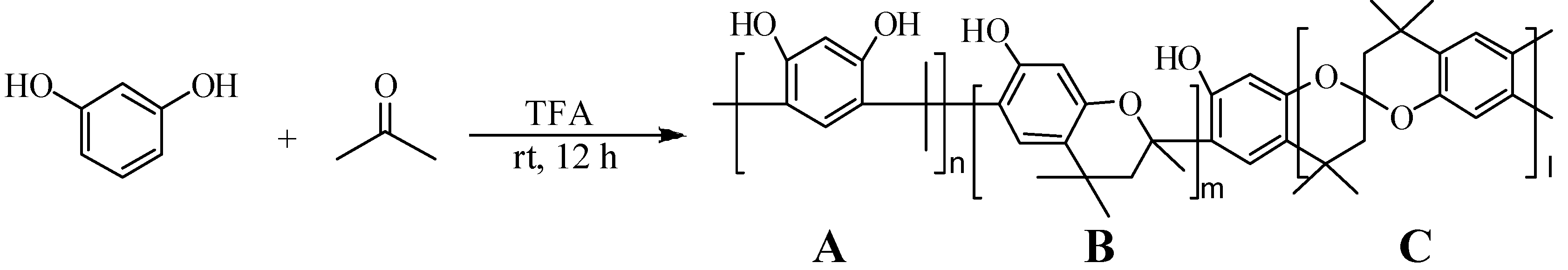
Synthesis
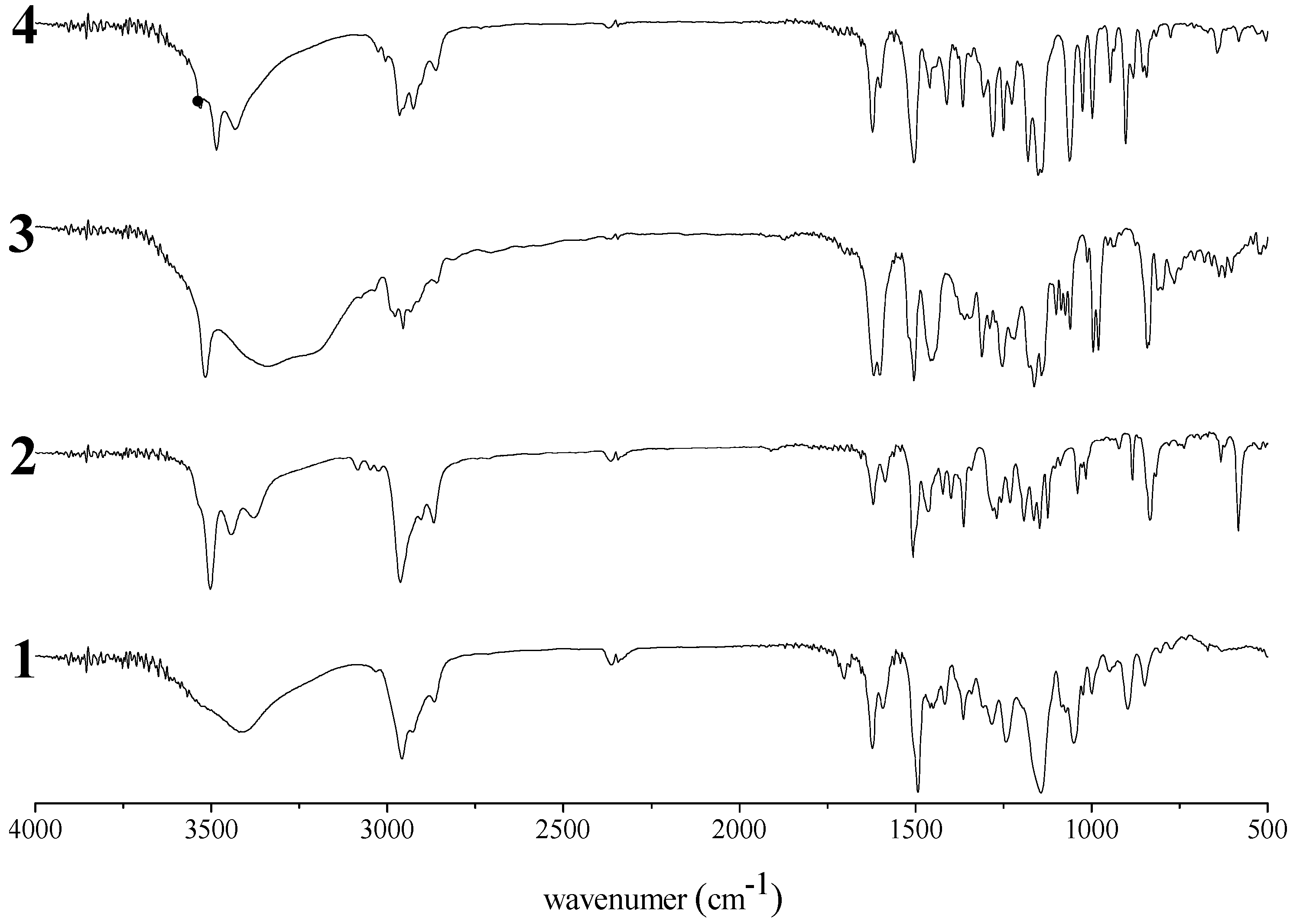
MALDI-TOF mass study
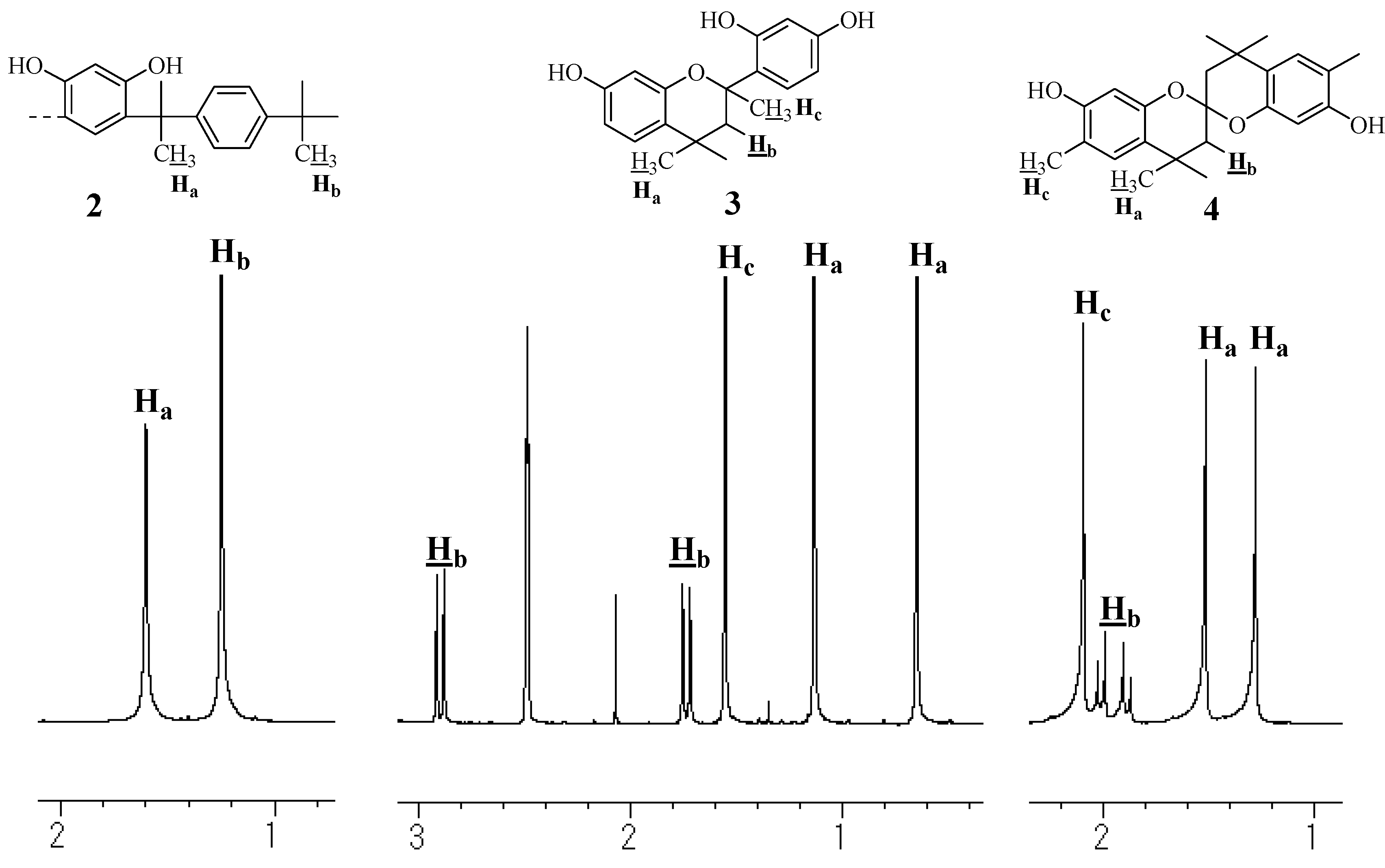
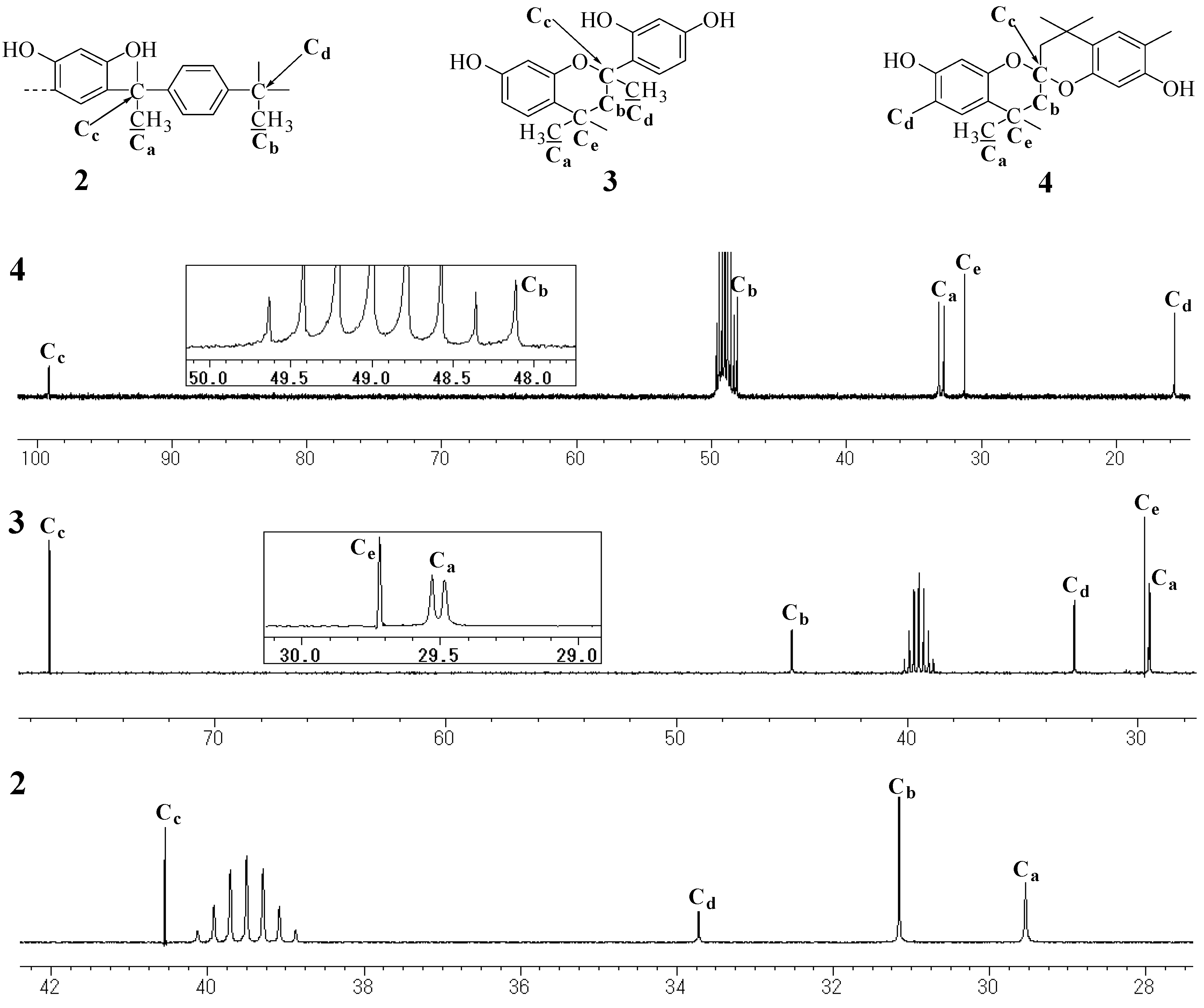
Model compounds and assignments
Unit A: isopropylidene bridge
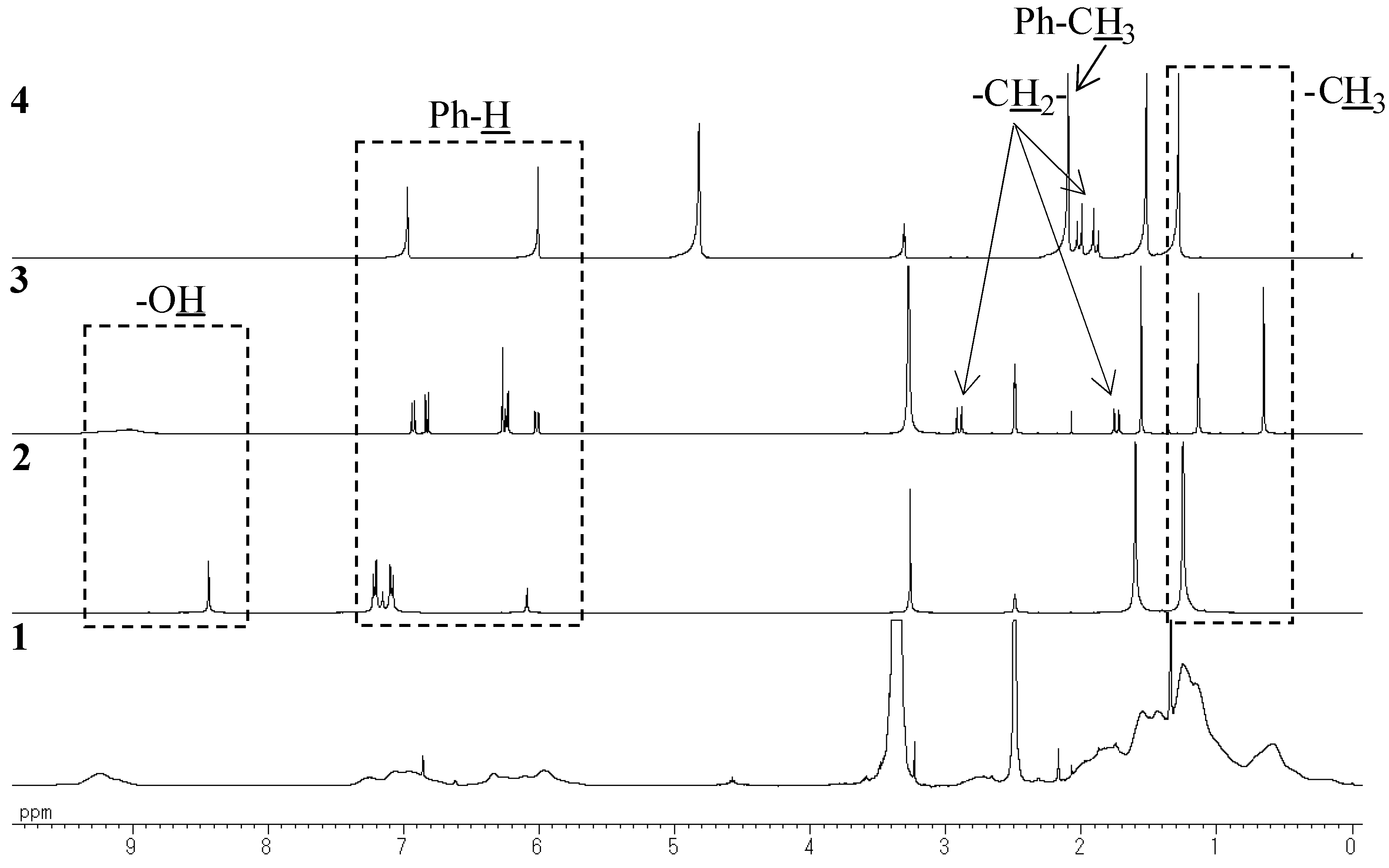
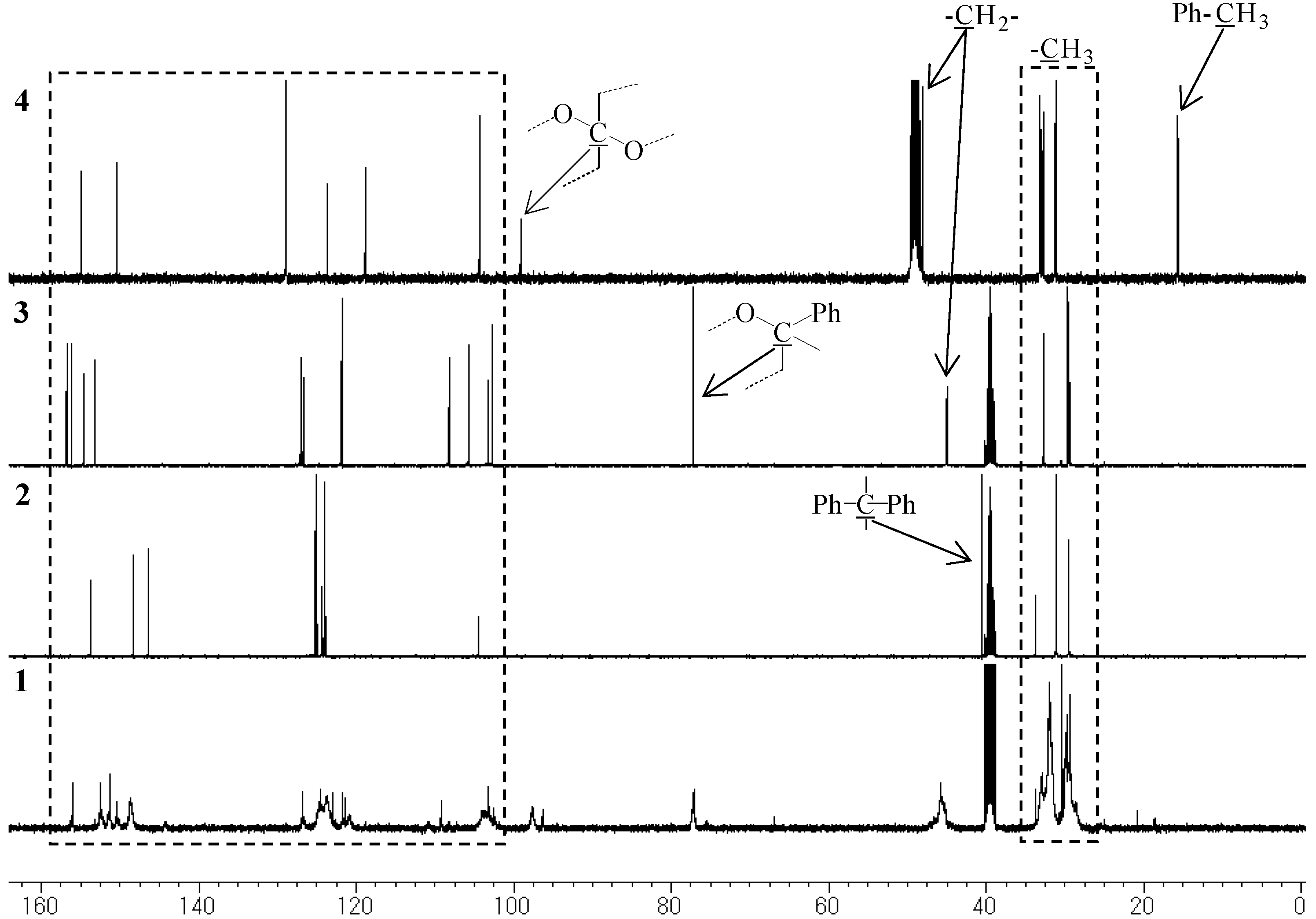
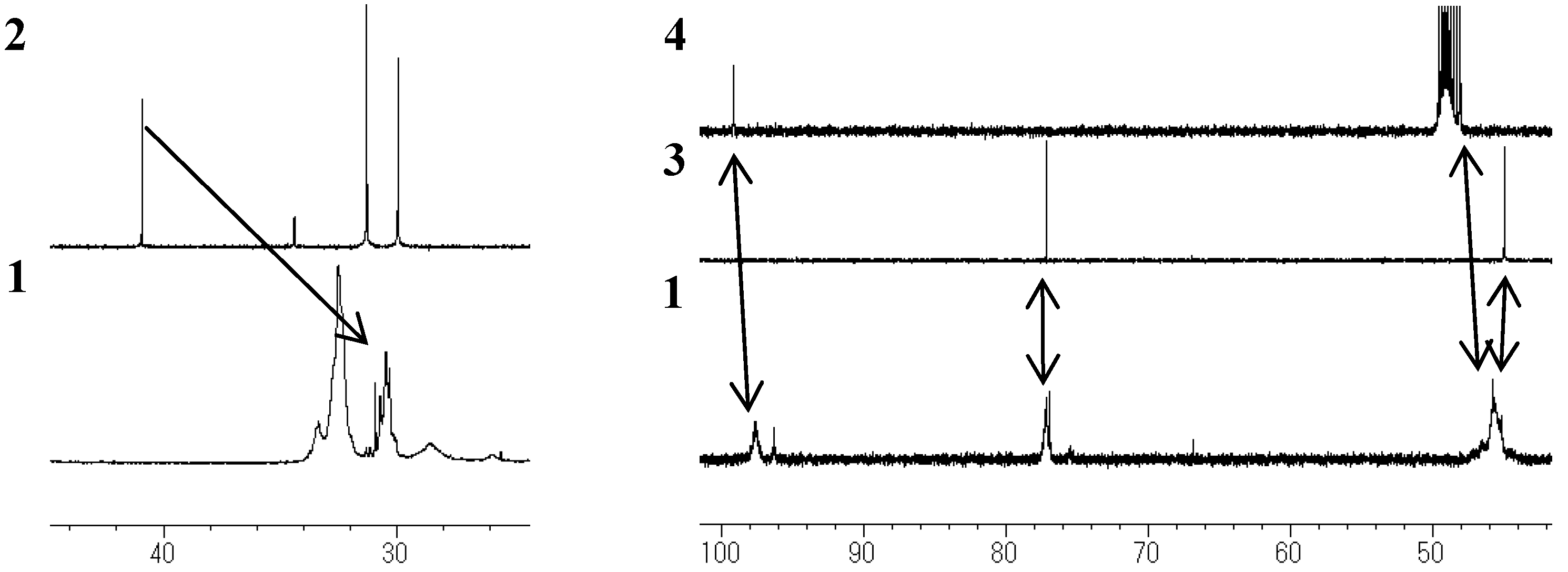
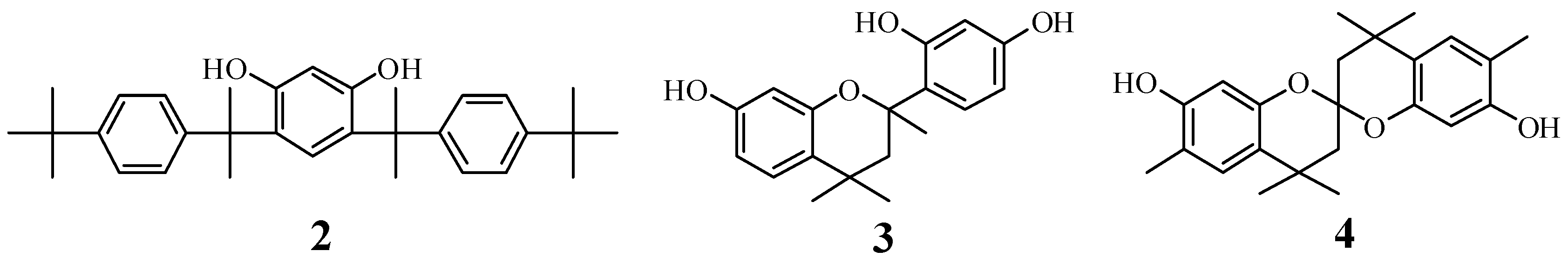
Unit B: chromane ring; Unit C: spiro-shaped double chromane ring

Content of phenolic hydroxyl group
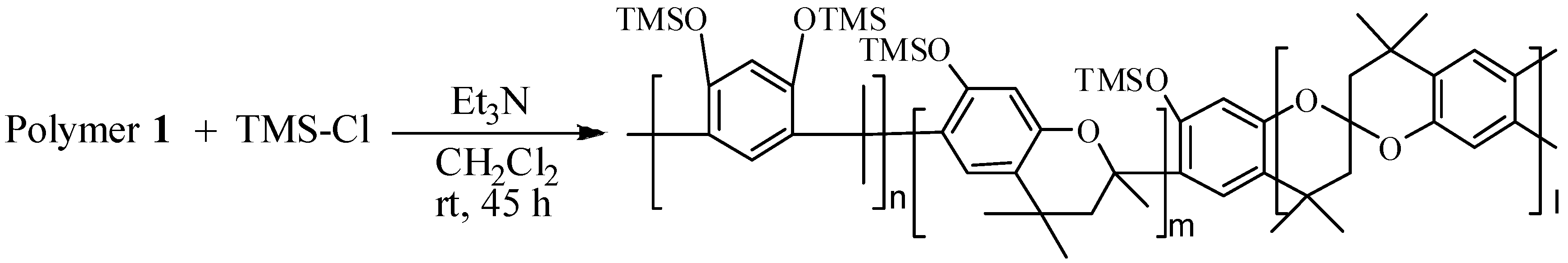
High molecular weight polymer
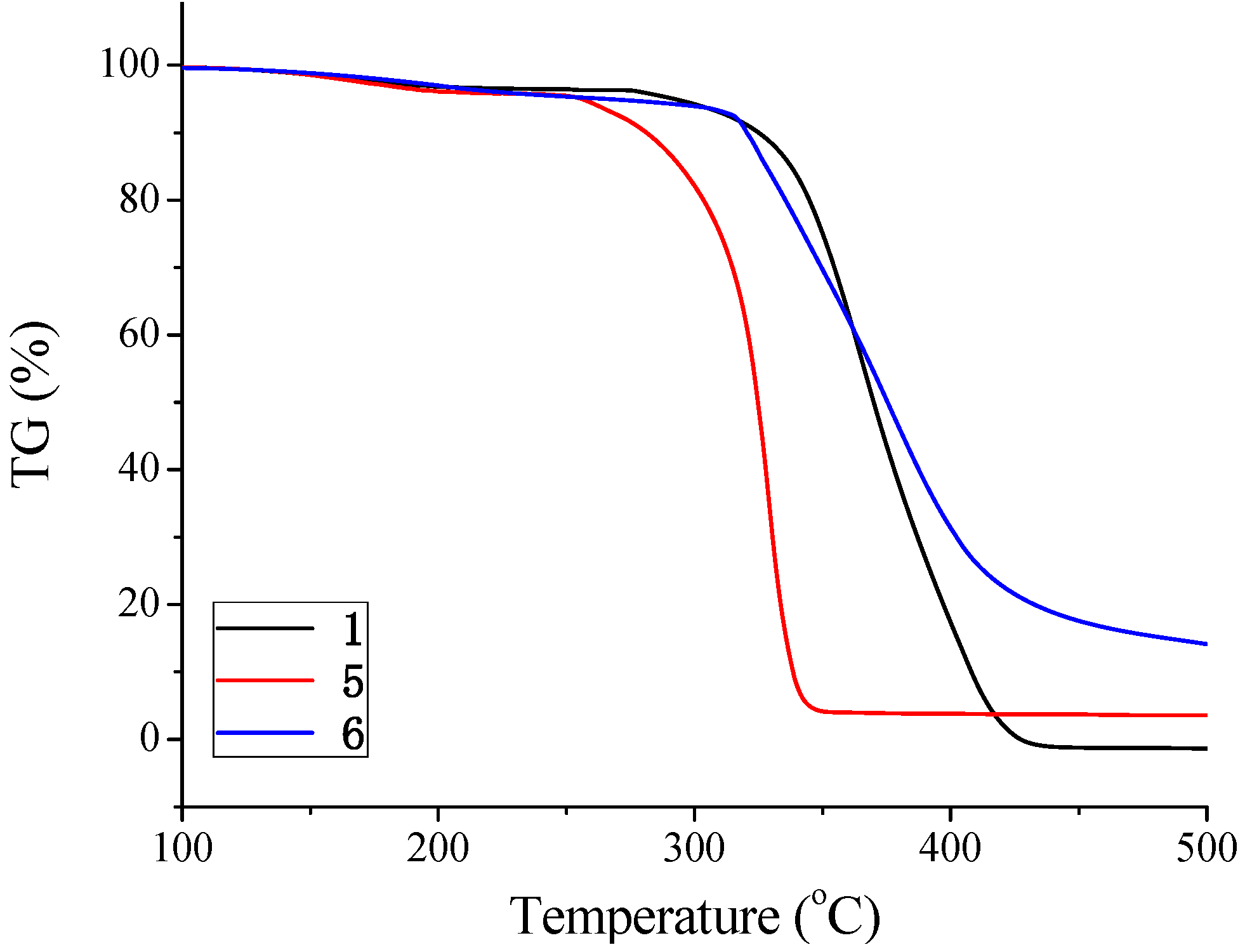
Thermal properties
Conclusions
Experimental
General
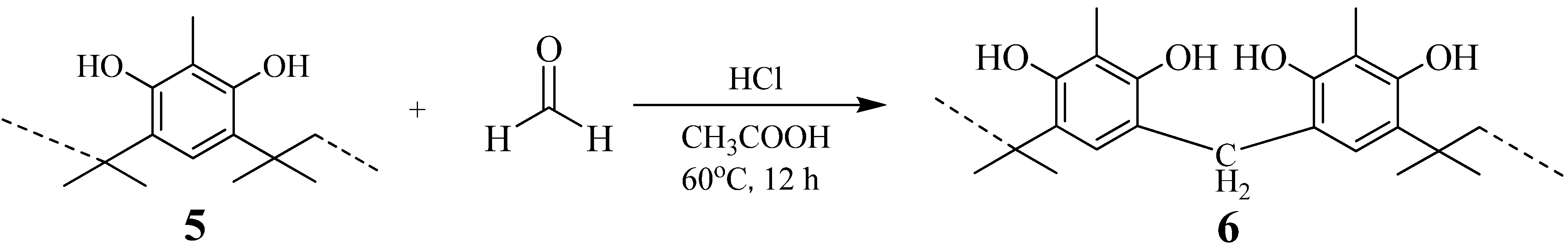
Preparation of resorcinol-acetone copolymer 1 and 2-methylresorcinol-acetone copolymer 5
Syntheses of Model Compound 2 (Scheme 4)

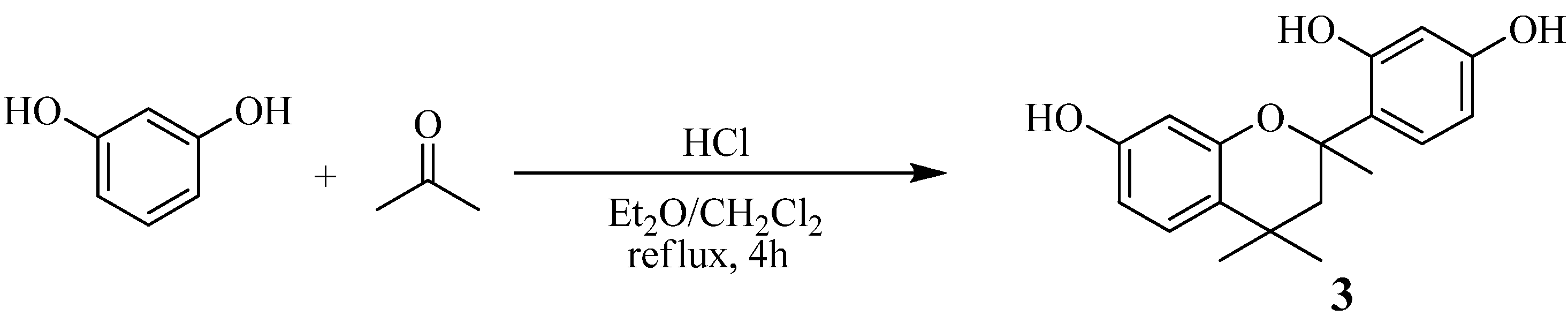
Synthesis of model compound 3 (Scheme 5)
Synthesis of model compound 4 (Scheme 6)
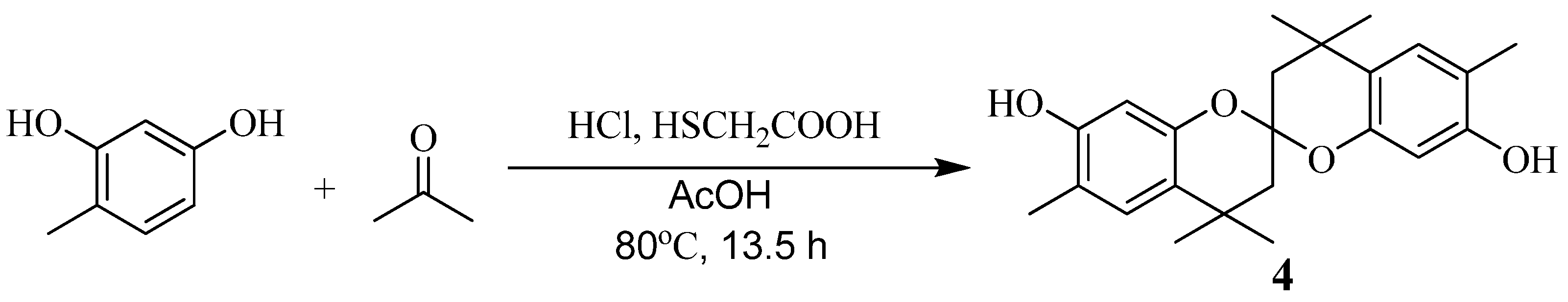
Preparation of high molecular weight polymer 6
TMS-modified polymer
Acknowledgements
References and Notes
- Konishi, G. Precision polymerization of designed phenol: new aspects of phenolic resin chemistry. J. Syn. Org. Chem. Jpn. 2008, 66, 705–713. [Google Scholar] [CrossRef]
- Timmerman, P.; Verboom, W.; Reinhoudt, D.N. Resorcinarenes. Tetrahedron 1996, 52, 2663–2704. [Google Scholar] [CrossRef]
- Bohmer, V. Calixarenes, macrocycles with (almost) unlimited possibilities. Angew. Chem. Inr. Ed. Engl 1995, 34, 713–745. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Aoyama, Y.; Tanaka, Y.; Toi, H.; Ogoshi, H. Polar host guest interaction – binding of nonionic polar compounds with a resorcinol-aldehyde cyclooligomer as a lipophilic polar host. J. Am. Chem. Soc. 1988, 110, 634–635. [Google Scholar] [CrossRef]
- Inouye, M.; Hashimoto, K.; Isagawa, K. Nondestructive Detection of Acetylcholine in Protic Media: Artificial-Signaling Acetylcholine Receptors. J. Am. Chem. Soc. 1994, 116, 5517–5518. [Google Scholar] [CrossRef]
- Wang, H.L.; Toppare, L.; Fernandes, J.E. Conducting polymer blends – polythiophene and polypyrole blends with polystyrene and poly(bisphenol-A carbonate). Macromolecules 1990, 23, 1053–1059. [Google Scholar] [CrossRef]
- Varnell, D.F.; Runt, J.P.; Coleman, M.M. Fourier-transform infrared studies of polymer blends 6. further observations on the poly(bisphenol-A carbonate)-poly(epsilon-caprolactone) system. Macromolecules 1981, 14, 1350–1356. [Google Scholar] [CrossRef]
- Nemoto, T.; Konishi, G.; Arai, T.; Takata, T. Synthesis and Properties of Fluorine-Containing Poly(arylenemethylene) as a New Heat-Resistant Denatured Phenolic Resin. Polym. J. 2008, 40, 622–628. [Google Scholar] [CrossRef]
- Livant, P.; Webb, T.R.; Weizheng, Xu. Reaction of resorcinol with acetone. J. Org. Chem. 1997, 62, 737–742. [Google Scholar] [CrossRef]
- Kadota, J.; Fukuoka, T.; Uyama, H.; Hasegawa, K.; Kobayashi, S. New positive-type photoresists based on enzymatically synthesized polyphenols. Macromol. Rapid Commun. 2004, 25, 441–444. [Google Scholar] [CrossRef]
- Jeerupan, J.; Konishi, G.; Nemoto, T.; Shin, D.-M.; Nakamoto, Y. Synthesis of Multifunctional Poly(calix[4]resorcinarene). Polym. J. 2007, 39, 762–763. [Google Scholar] [CrossRef]
- Nemoto, T.; Ueno, T.; Nishi, M.; Shin, D.-M.; Nakamoto, Y.; Konishi, G. Synthesis and Properties of Organosoluble Poly(phenylenemethylene)s from Substituted Benzenes or Naphthalenes. Polym. J. 2006, 38, 1278–1282. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, Y.; Konishi, G. Preparation of Organic/Inorganic Polymer Hybrid from a New Class of Novolac. Polym. J. 2006, 38, 606–609. [Google Scholar] [CrossRef]
- Chung, J.E.; Kurisawa, M.; Kim, Y.J.; Uyama, H.; Kobayashi, S. Amplification of antioxidant activity of aatechin by polycondensation with acetaldehyde. Biomacromolecules 2004, 5, 113–118. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Curing Behaviors of Crosslinkable Polynaphthols from Renewable Resources: Preparation of Artificial Urushi. Macromolecules 2004, 37, 1777–1782. [Google Scholar] [CrossRef]
- Turkmen, N.; Velioglu, Y.S.; Sari, F.; Polat, G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activity of black tea. Molecules 2007, 12, 484–496. [Google Scholar] [CrossRef]
- Kubo, I.; Nitoda, T.; Nihei, K. Effects of Quercetin on Mushroom Tyrosinase and B16-F10 Melanoma Cells. Molecules 2007, 12, 1045–1056. [Google Scholar] [CrossRef]
- Kadoma, Y.; Fujisawa, S. A Comparative Study of the Radical-scavenging Activity of the Phenolcarboxylic Acids Caffeic Acid, p-Coumaric Acid, Chlorogenic Acid and Ferulic Acid, With or Without 2-Mercaptoethanol, a Thiol, Using the Induction Period Method. Molecules 2008, 13, 2488–2499. [Google Scholar] [CrossRef]
- Mao, Z.; Zheng, Y.L.; Zhang, Y.Q.; Han, B.P.; Zhu, X.W.; Chang, Q.; Hu, X.B. The Anti-apoptosis Effects of Daidzein in the Brain of D-Galactose Treated Mice. Molecules 2007, 12, 1455–1470. [Google Scholar] [CrossRef]
- Yang, D.P.; Ji, H.F.; Tang, G.Y.; Ren, W.; Zhang, H.Y. How Many Drugs Are Catecholics. Molecules 2007, 12, 878–884. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y. Litchi Flavonoids: Isolation, Identification and Biological Activity. Molecules 2007, 12, 745–758. [Google Scholar] [CrossRef]
- Mikame, K.; Funaoka, M. Polymer Structure of Lignophenol I —Structure and Function of Fractionated Lignophenol—. Polym. J. 2006, 38, 585–591. [Google Scholar] [CrossRef]
- Mikame, K.; Funaoka, M. Polymer Structure of Lignophenol II —Comparison of Molecular Morphology of Lignophenol and Conventional Lignins—. Polym. J. 2006, 38, 592–596. [Google Scholar] [CrossRef]
- Mikame, K.; Funaoka, M. Conversion and Separation Pattern of Lignocellulosic Carbohydrates through the Phase-separation System. Polym. J. 2006, 38, 694–702. [Google Scholar] [CrossRef]
- Sample Availability: Available from GK.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kobayashi, A.; Konishi, G.-i. Synthesis and Analysis of Resorcinol-Acetone Copolymer. Molecules 2009, 14, 364-377. https://doi.org/10.3390/molecules14010364
Kobayashi A, Konishi G-i. Synthesis and Analysis of Resorcinol-Acetone Copolymer. Molecules. 2009; 14(1):364-377. https://doi.org/10.3390/molecules14010364
Chicago/Turabian StyleKobayashi, Ataru, and Gen-ichi Konishi. 2009. "Synthesis and Analysis of Resorcinol-Acetone Copolymer" Molecules 14, no. 1: 364-377. https://doi.org/10.3390/molecules14010364