Synthesis of Novel Homo-N-Nucleoside Analogs Composed of a Homo-1,4-Dioxane Sugar Analog and Substituted 1,3,5-Triazine Base Equivalents
Abstract
:Introduction
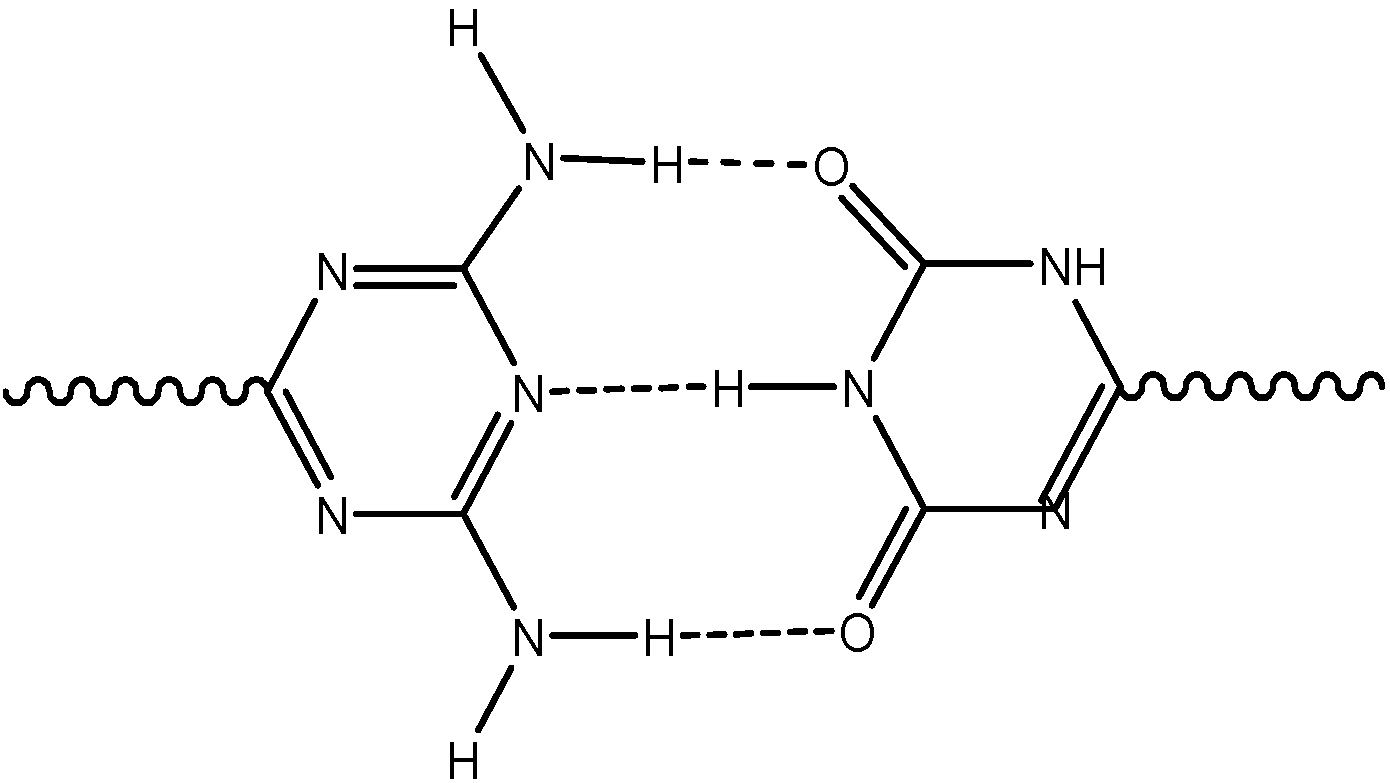

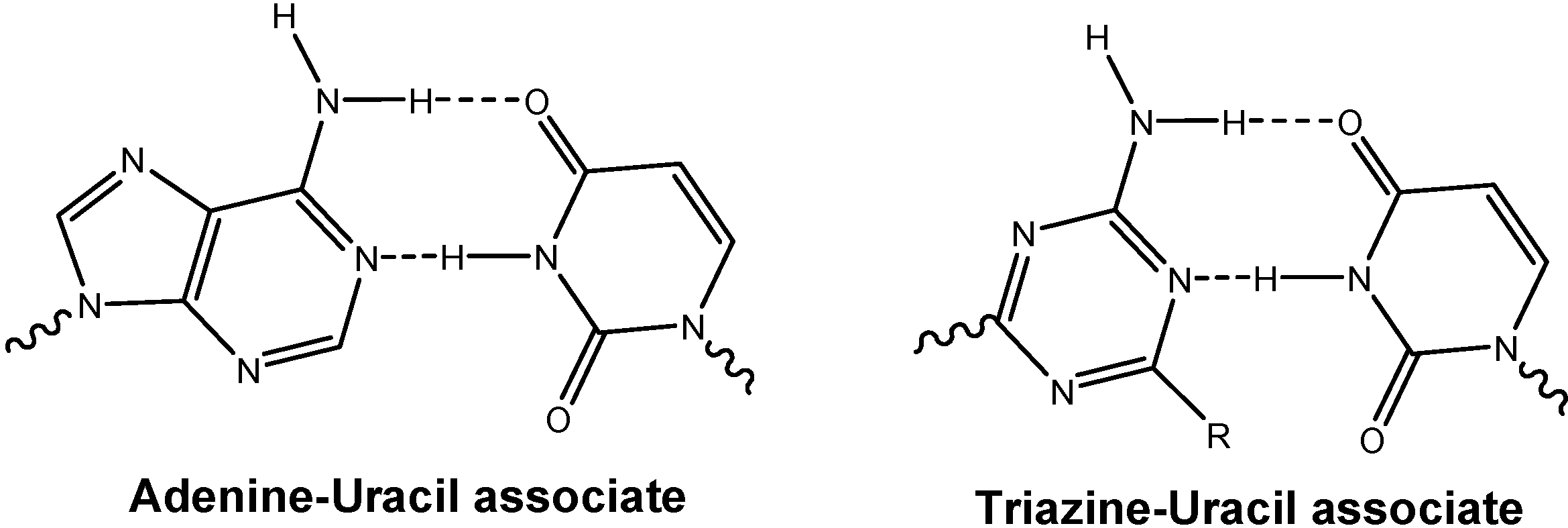
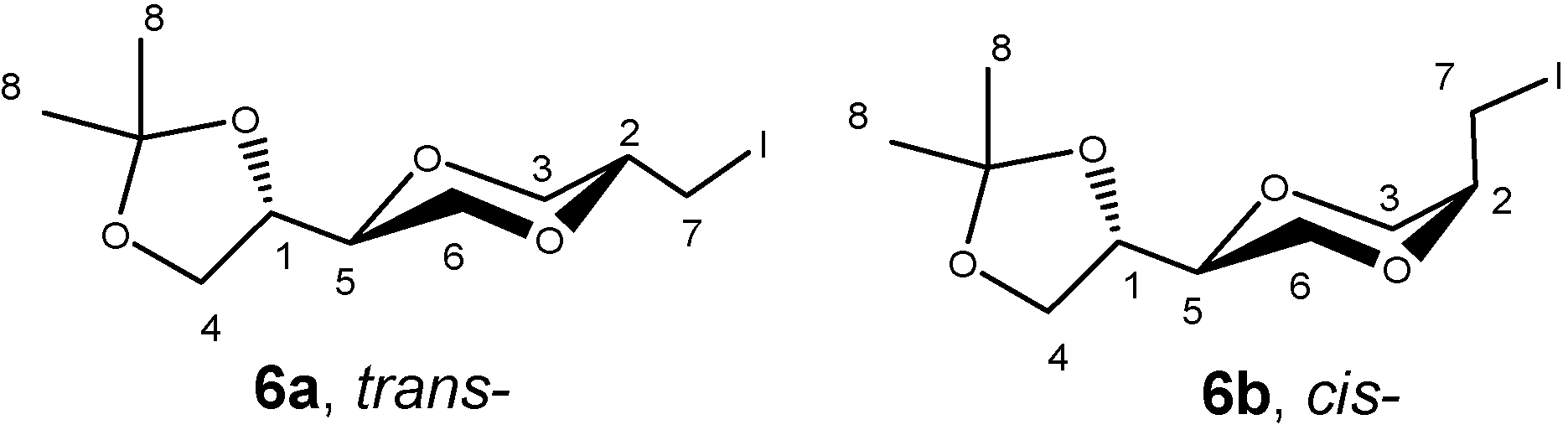
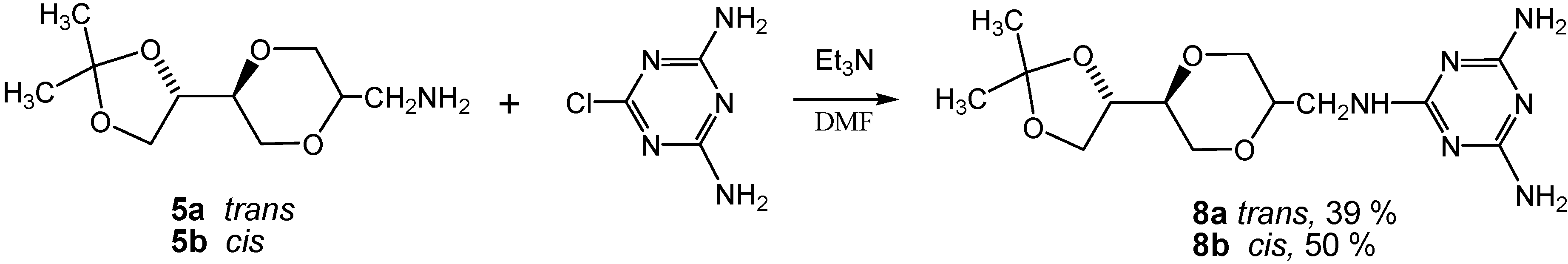
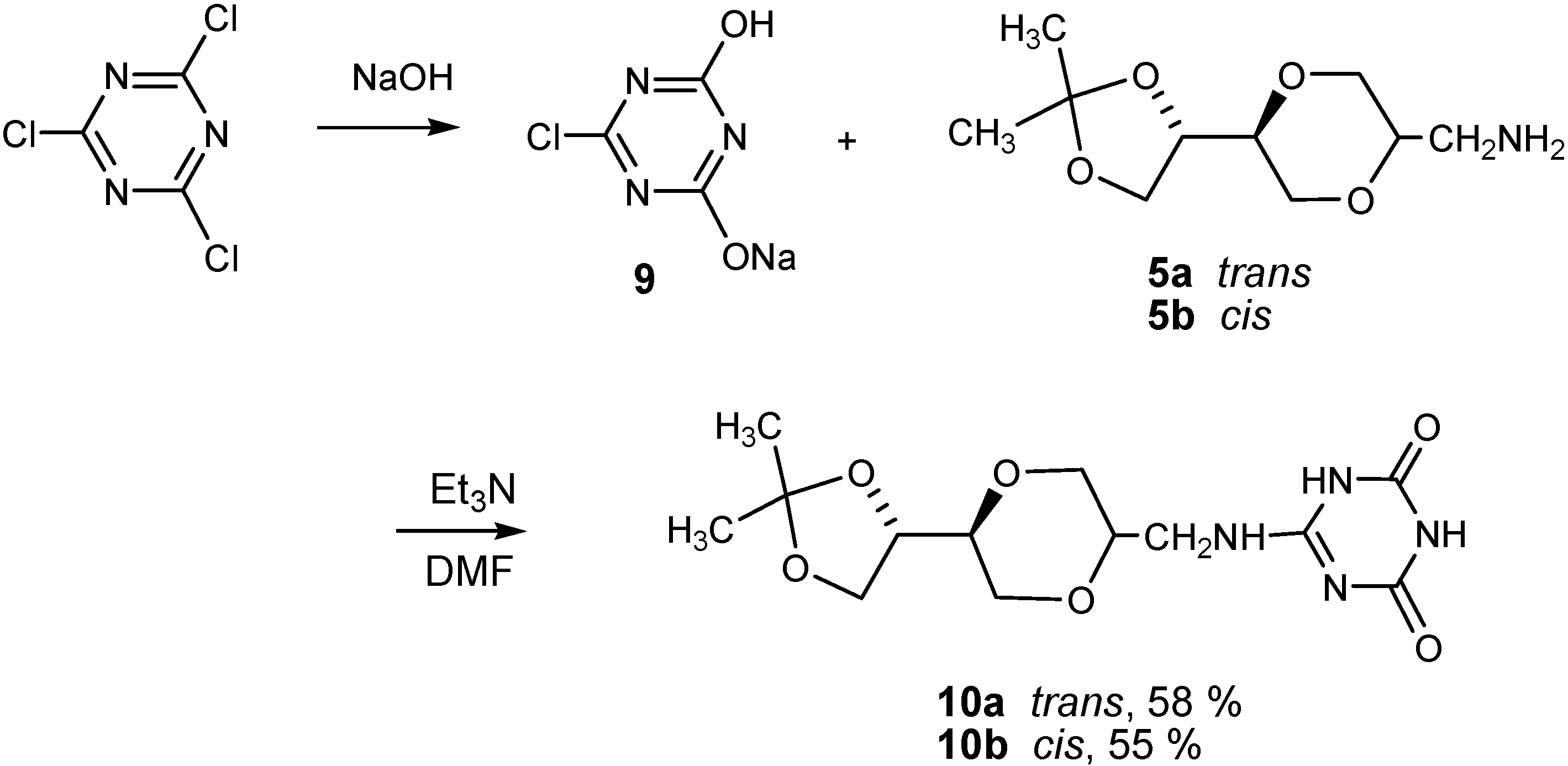
Results and Discussion


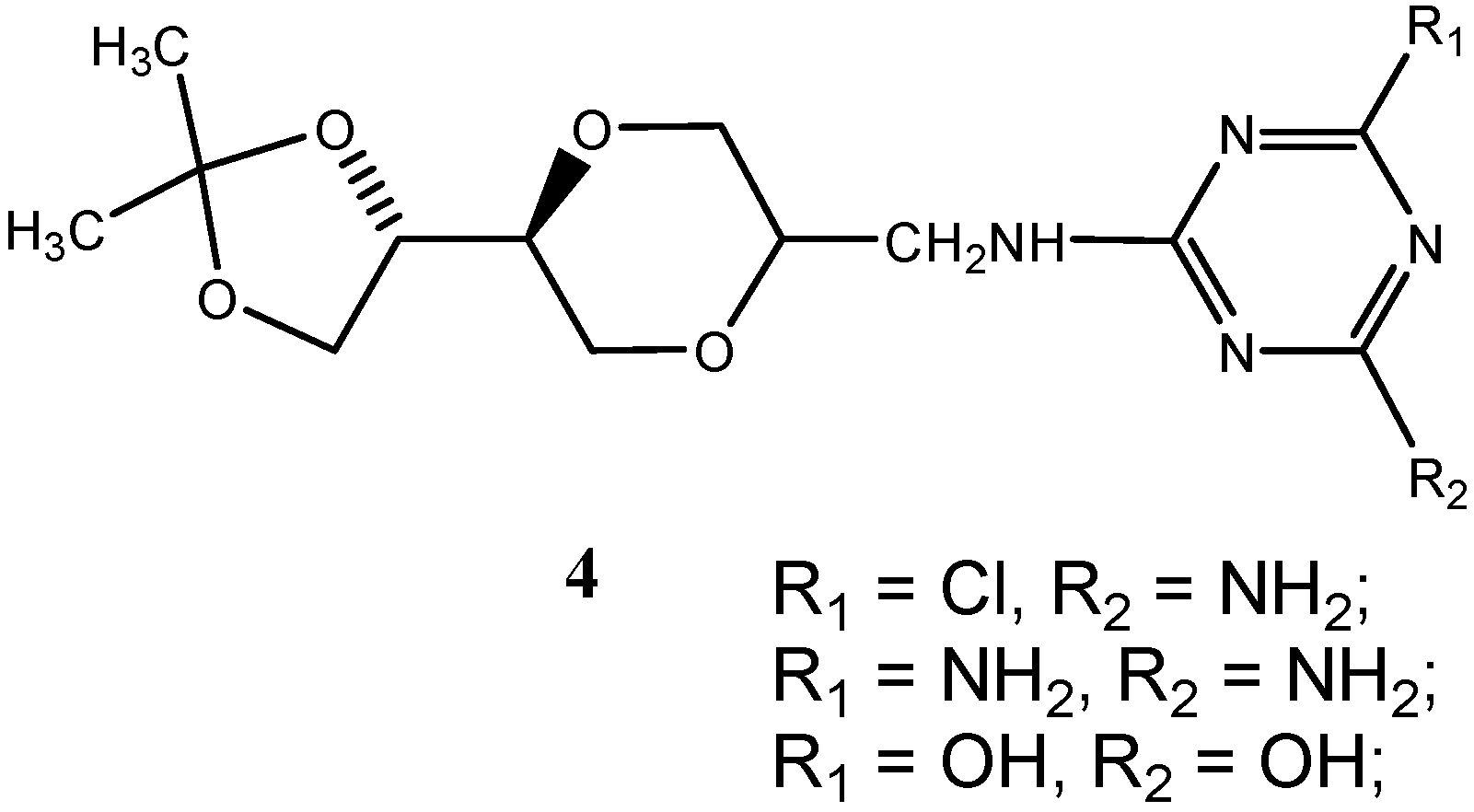
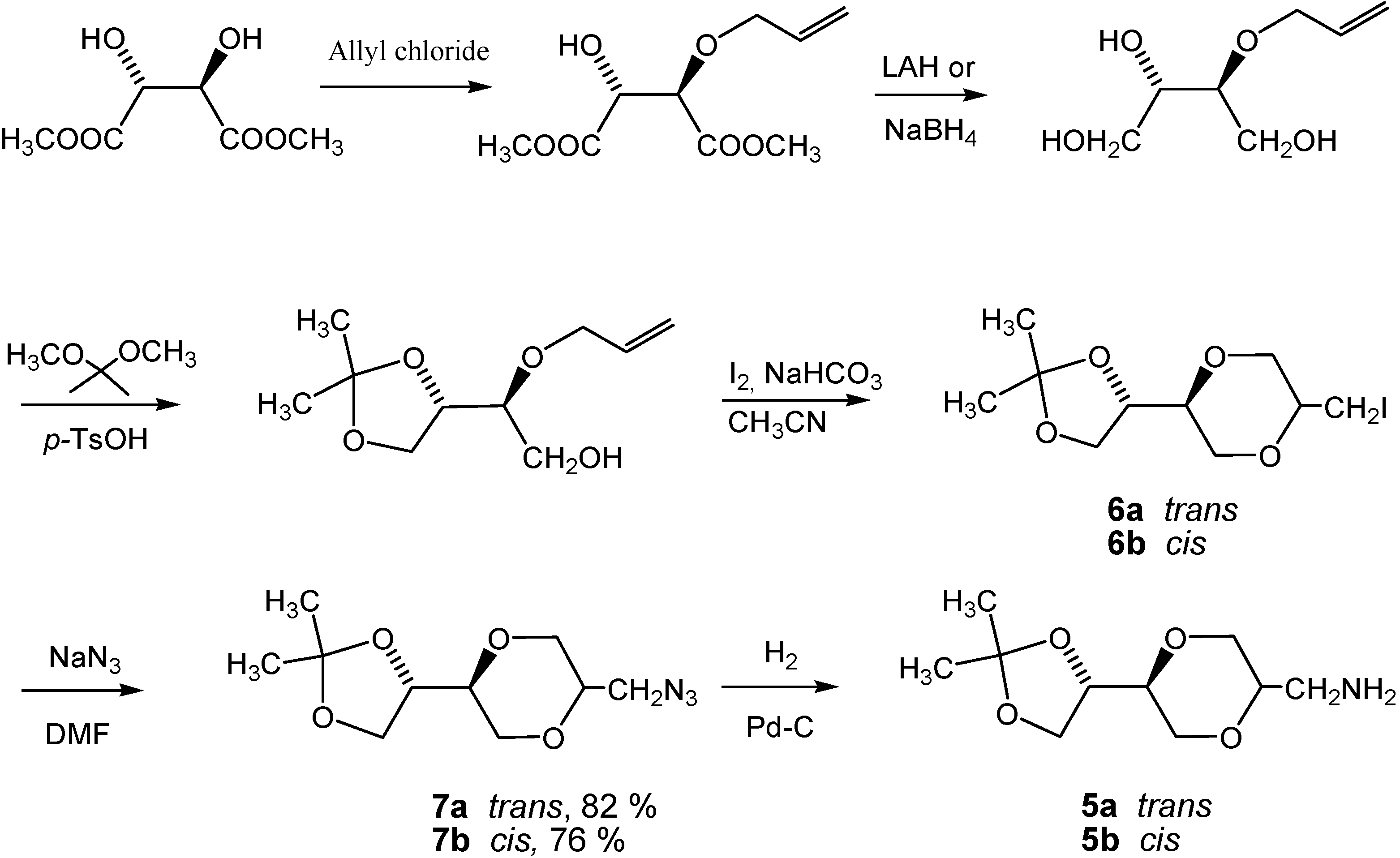

Conclusions
Experimental
General











Acknowledgements
References
- Li, F.; Maag, H.; Alfredson, T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J. Pharm. Sci. 2007, 97, 1109–1134. [Google Scholar]
- Adrian, R.; Murakami, E.; Basavapathruni, A.; Vaccaro, J.A.; Ulrich, D.; Chu, C.K.; Schinazi, R.F.; Anderson, K.S. Probing the molecular mechanisms of AZT drug resistance mediated by HIV-1 reverse transcriptase using a transient kinetic analysis. Biochemistry 2003, 42, 8831–8841. [Google Scholar] [CrossRef]
- Ren, J.; Esnouf, R.M.; Hopkins, A.L.; Jones, E.Y.; Kirby, I.; Keeling, J.; Ross, C.K.; Larder, B.A.; Stuart, D.I.; Stammers, D.K. 3'-Azido-3'-deoxythymidine drug resistance mutations in HIV-1 reverse transcriptase can induce long range conformational changes. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 9518–9523. [Google Scholar] [CrossRef]
- Feng, J.Y.; Anderson, K.S. Mechanistic Studies Examining the Efficiency and Fidelity of DNA Synthesis by the 3TC-Resistant Mutant (184V) of HIV-1 Reverse Transcriptase. Biochemistry 1999, 38, 9440–9448. [Google Scholar] [CrossRef]
- Hayatsu, H. Orgueil Meteorite: Organic Nitrogen Contents. Science 1964, 146, 1291–1293. [Google Scholar]
- Hayatsu, R.; Studier, M.H.; Moore, L.P.; Anders, E. Purines and triazines in the Murchison meteorite. Geochim. Cosmochim. Acta 1975, 39, 471–488. [Google Scholar] [CrossRef]
- Minard, R.D.; Hatcher, P.G.; Gourley, R.C.; Matthews, C.N. Structural investigations of hydrogen cyanide polymers: new insights using TMAH thermochemolysis/GC-MS, Origins of life and evolution of the biosphere. J. International Soc. Study Orig. Life 1998, 28, 461–473. [Google Scholar]
- Hysell, M.; Siegel, J.S.; Tor, Y. Synthesis and stability of exocyclic triazine nucleosides. Org. Biomol. Chem. 2005, 3, 2946–295. [Google Scholar] [CrossRef]
- Blotny, G. Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Kosary, J.; Kasztreiner, E.; Rabloczky, G.; Kurthy, M. Synthesis and cardiotonic activity of 2,4-diamino-1.3,5-triazines. Eur. J. Med. Chem. 1989, 24, 97–99. [Google Scholar] [CrossRef]
- Hargreaves, R.B.; McLoughlin, B.J.; Mills, S.D. Thiadiazine, oxadiazine and triazine derivatives, and pharmaceutical compositions containing them. Eur. Pat. Appl. 1983. EP 85227. [Google Scholar]
- Kukla, M.J.; Ludovici, D.W.; Janssen, P.A.J.; Heeres, J.; Moereels, H.E.L. Preparation and anti-HIV activity of substituted diamino-1,3,5-triazine derivatives. Eur. Pat. Appl. 1998. EP 834507. [Google Scholar]
- De Corte, B.; De Jonge, M.R.; Heeres, J.; Janssen, P.A.J; Koymans, L.M.H.; Kukla, M.J.; Ludovici, D.W.; Van Aken Koen, J.A. Preparation of 2,4-disubstituted triazine derivatives as anti - HIV agents. PCT Int. Appl. 2000. WO 2000027828. [Google Scholar]
- Brzozowski, Z.; Saczewski, F.; Gdaniec, M. Synthesis, structural characterization and antitumor acitivity of novel 2,4-diamino-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2000, 35, 1053–1064. [Google Scholar] [CrossRef]
- An, H.; Chamakura, V.; Chen, H.; Hong, Z. Solid phase synthesis and combinatorial library of nucleosides as potential antiviral and anticancer agents. PCT Int. Appl. 2003. WO 2003051898. [Google Scholar]
- Ames, M.M. Hexamethylmelamine: pharmacology and mechanism of action. Cancer treatment rev. 1991, 18, Suppl. A. 3–14. [Google Scholar] [CrossRef]
- Varaprasad, C.V.; Habib, Q.; Li, D.Y.; Huang, J. Synthesis of novel exocyclic amino nucleosides by parallel solid-phase combinatorial strategy. Tetrahedron 2003, 59, 2297–2307. [Google Scholar] [CrossRef]
- Gaubert, G.; Gosselin, G.; Eriksson, S.; Vita, A.; Maury, G. Unnatural enantiomers of 5-azacytidine analogues: syntheses and enzymatic properties. Nucleosides Nucleotides Nucleic Acids 2001, 20, 837–840. [Google Scholar] [CrossRef]
- Archer, E.A.; Goldberg, N.T.; Lynch, V.; Krische, M.J. Nanostructured Polymer Duplexes via the Covalent Casting of 1-Dimensional H-Bonding Motifs: A New Strategy for the Self-Assembly of Macromolecular Precursors. J. Am. Chem. Soc. 2000, 122, 5006–5007. [Google Scholar] [CrossRef]
- Archer, E.A.; Cauble, D.F.; Lynch, V.; Krische, M.J. Synthetic duplex oligomers: optimizing interstrand affinity through the use of a non-covalent constraint. Tetrahedron 2002, 58, 721–725. [Google Scholar] [CrossRef]
- Löwik, D.W.P.M.; Lowe, C.R. Synthesis of macrocyclic, triazine-based receptor molecules. Eur. J. Org. Chem. 2001, 15, 2825–2839. [Google Scholar]
- Choi, I.S.; Li, X.; Simanek, E.E.; Akaba, R.; Whitesides, G.M. Self-Assembly of Hydrogen-Bonded Polymeric Rods Based on the Cyanuric Acid Melamine Lattice. Chem. Mat. 1999, 11, 684–690. [Google Scholar] [CrossRef]
- Shiki, Y.; Takashi, K.; Akihide, K. Melamine-barbiturate/cyanurate binary organogels possessing rigid azobenzene-tether moiety. Langmuir 2005, 21, 11048–11052. [Google Scholar] [CrossRef]
- Kunz, M.J.; Hayn, G.; Saf, R.; Binder, W.H. Hydrogen-bonded supramolecular poly(ether ketones). J. Polym. Sci., A: Polym. Chem. 2004, 42, 661–674. [Google Scholar] [CrossRef]
- Asanuma, H.; Ban, T.; Gotoh, S.; Hishiya, T.; Komiyama, M. Precise recognition of nucleotides and their derivatives through hydrogen bonding in water by poly(vinyldiaminotriazine). Supramol. Sci. 1998, 5, 405–410. [Google Scholar] [CrossRef]
- Nie, L.; Ma, H.; Li, X.; Sun, M.; Xiong, S. Recognition of thymine by triazine fluorescent probe through intermolecular multiple hydrogen bonding. Biopolymers 2003, 72, 274–281. [Google Scholar] [CrossRef]
- Jochims, J.C.; Von, V.; Hubertus, W.G. Barriers to hindered rotation around the N-glycosidic bond, I. N-Glucopyranosides. Chem. Ber. 1978, 111, 1693–1708. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Z.; Su, X.; Fu, L.; Qu, F. Synthesis of 2,4-dioxohexahydro-1,3,5-triazine glucosides. J. Wuhan Univ. Nat. Sci. Ed. 1999, 45, 168–170. [Google Scholar]
- Simmonds, R.J.; Stevens, M.F.G. Triazines and related products. Part 25. Methods for the attachment of sugar residues to cytotoxic 1,3,5-triazines. J. Chem. Soc., Perkin Trans. 1, Org. Bioorg. Chem. 1982, 8, 1821–1825. [Google Scholar] [CrossRef]
- Huchel, U.; Schmidt, C.; Schmidt, R.R. Anomeric O-alkylation. Part 16. Synthesis of hetaryl glycosides and their glycosyl donor properties. Eur. J. Org. Chem. 1998, 7, 1353–1360. [Google Scholar]
- Niedballa, U.; Vorbruggen, H. A general synthesis of N-glycosides. V. Synthesis of 5-azacytidines. J. Org. Chem. 1974, 39, 3672–3674. [Google Scholar]
- Yu, Q.; Carlsen, P. Synthesis of Novel, Optically Active Uridine Analog Containing a 1,4-Dioxane Sugar Moiety. Synthesis of the Corresponding Dinucleotide. Nucleos. Nucleot. Nucleic Acids. Submitted.
- Hossain, N.; Blaton, N.; Peeters, O.; Rozenski, J.; Herdewijn, P.A. Synthesis of homo-N- nucleosides, a series of C1' branched-chain nucleosides. Tetrahedron 1996, 52, 5563–5578. [Google Scholar]
- Hossain, N.; Hendrix, C.; Lescrinier, E.; Van Aerschot, A.; Busson, R.; De Clercq, E.; Herdewijn, P. Homo-N - nucleosides : incorporation into oligonucleotides and antiviral activity. Bioorg. Med. Chem. Lett. 1996, 6, 1465–1468. [Google Scholar] [CrossRef]
- Franzyk, H.; Rasmussen, J.H.; Mazzei, R.A.; Jensen, S.R. Synthesis of carbocyclic homo - N - nucleosides from iridoids. Eur. J. Org. Chem. 1998, 12, 2931–2935. [Google Scholar]
- Chun, M.W.; Kim, J.H.; Kim, M.J.; Kim, B.R.; Jeong, L.S. Synthesis of homo-N-nucleoside with 1,2,4-triazole-3-carboxamide. Nucleos. Nucleot. Nucleic Acids 2005, 24, 979–981. [Google Scholar] [CrossRef]
- Saladino, R.; Ciambecchini, U.; Hanessian, S. Synthesis of 1'- homo-N-nucleosides from hexitols. Eur. J. Org. Chem. 2003, 22, 4401–4405. [Google Scholar]
- Asanuma, H.; Ban, T.; Gotoh, S.; Hishiya, T.; Komiyama, M. Precise recognition of nucleotides and their derivatives through hydrogen bonding in water by poly(vinyldiaminotriazine). Supramol. Sci. 1998, 5, 405–410. [Google Scholar] [CrossRef]
- Appeldoorn, C.C.M.; Joosten, J.A.F.; Ait el Maate, F.; Dobrindt, U.; Hacker, J.; Liskamp, R.J.; Khan, A.; Pieters, R.J. Novel multivalent mannose compounds and their inhibition of the adhesion of type 1 fimbriated uropathogenic E. coli. Tetrahedron Asymmetry 2005, 16, 361–372. [Google Scholar]
- Rye, C.S.; Withers, S.G. Synthesis and evaluation of potential inhibitors of chondroitin AC Lyase from Flavobacterium heparinum. J. Org. Chem. 2002, 67, 4505–4512. [Google Scholar] [CrossRef]
- Yu, Q.; Carlsen, P. Enantioselective Synthesis of Homo-N-Nucleosides Containing a 1,4-Dioxane Sugar Analog. Molecules 2008, 13, 2962–2974. [Google Scholar] [CrossRef] [Green Version]
- Horrobin, B.S. The hydrolysis of some chloro-1,3,5-triazines: Mechanism: Structure and Reactivity. J. Chem. Soc. 1963, 4130–4145. [Google Scholar] [CrossRef]
- Baliani, A.; Bueno, G.J.; Stewart, M.L.; Yardley, V.; Brun, R.; Barrett, M.P.; Gilbert, L.H. Design and Synthesis of a Series of Melamine-based Nitroheterocycles with Activity against Trypanosomatid Parasites. J. Med. Chem. 2005, 48, 5570–5575. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Oniciu, D.C.; Ghiviriga, I.; Barcock, R.A. 4,6-Bis- and 2,4,6-tris-(N, N-dialkylamino)-s-triazines: synthesis, NMR spectra and restricted rotations. J. Chem. Soc. Perkin Trans. 2: Phys. Org. Chem. 1995, 4, 785–792. [Google Scholar]
- Katritzky, A.R.; Ghiviriga, I.; Steel, P.J.; Oniciu, D.C. Restricted rotations in 4,6-bis- and 2,4,6-tris(N, N-dialkylamino)-s-triazines. J. Chem. Soc. Perkin Trans. 2: Phys. Org. Chem. 1996, 3, 443–447. [Google Scholar]
- Amm, M.; Platzer, N.; Guilhem, J.; Bouchet, J.; Volland, J. Structural and conformational study of substituted triazines by 15N NMR and x-ray analysis. Mag. Res. Chem. 1998, 36, 587–596. [Google Scholar] [CrossRef]
- Birkett, H.E.; Harris, R.K.; Hodgkinson, P.; Carr, K.; Charlton, M.H.; Cherryman, J.C.; Chippendale, A.M.; Glover, R.P. NMR studies of exchange between triazine rotamers. Mag. Res. Chem. 2000, 38, 504–511. [Google Scholar]
- Sample Availability: Samples of selected compounds are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, Q.; Schwidom, D.; Exner, A.; Carlsen, P. Synthesis of Novel Homo-N-Nucleoside Analogs Composed of a Homo-1,4-Dioxane Sugar Analog and Substituted 1,3,5-Triazine Base Equivalents. Molecules 2008, 13, 3092-3106. https://doi.org/10.3390/molecules13123092
Yu Q, Schwidom D, Exner A, Carlsen P. Synthesis of Novel Homo-N-Nucleoside Analogs Composed of a Homo-1,4-Dioxane Sugar Analog and Substituted 1,3,5-Triazine Base Equivalents. Molecules. 2008; 13(12):3092-3106. https://doi.org/10.3390/molecules13123092
Chicago/Turabian StyleYu, Qiang, Dirk Schwidom, Alexander Exner, and Per Carlsen. 2008. "Synthesis of Novel Homo-N-Nucleoside Analogs Composed of a Homo-1,4-Dioxane Sugar Analog and Substituted 1,3,5-Triazine Base Equivalents" Molecules 13, no. 12: 3092-3106. https://doi.org/10.3390/molecules13123092



