Pyrogallol Structure in Polyphenols is Involved in Apoptosis-induction on HEK293T and K562 Cells
Abstract
:Introduction
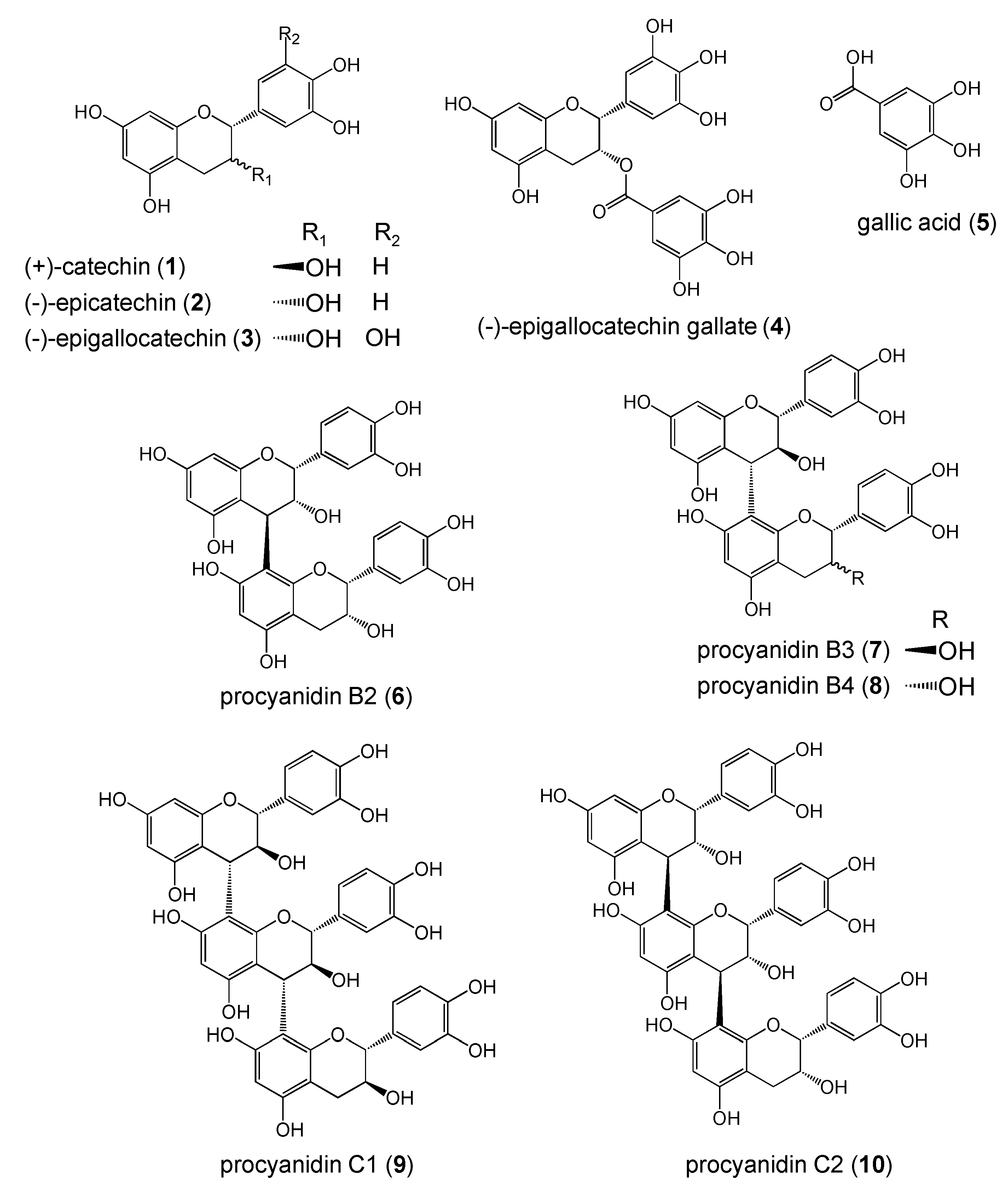
Results and Discussion
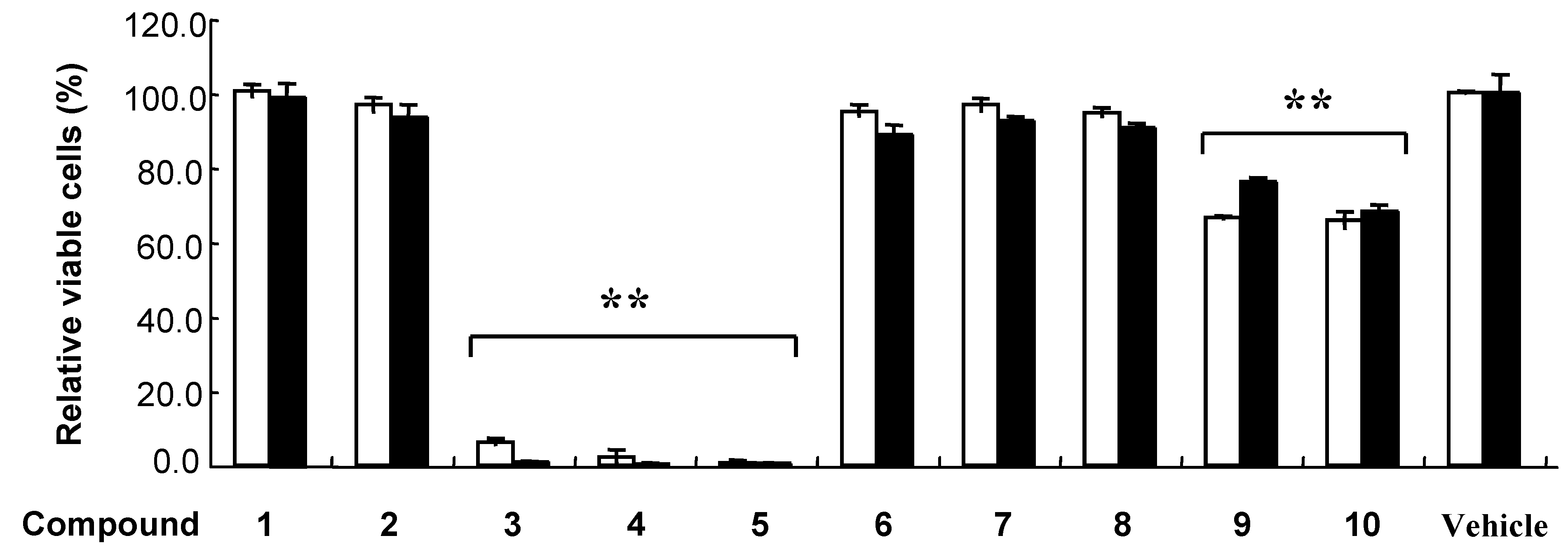
| K562 | HEK293T | |||||
| LC50 (μM) | LC50 (μM) | |||||
| Compound | 24 h | 48 h | LC50(24h)/LC50(48h) | 24h | 48h | LC50(24h)/LC50(48h) |
| 3 | 63.3 ± 3.2 | 33.0 ± 1.4 | 1.92 | 20.5 ± 3.0 | 17.2 ± 3.2 | 1.19 |
| 4 | 51.8 ± 2.5 | 36.2 ± 1.7 | 1.43 | 28.5 ± 3.7 | 27.3 ± 1.8 | 1.04 |
| 5 | 57.4 ± 7.9 | 43.9 ± 5.1 | 1.31 | 28.7 ± 2.1 | 29.2 ± 2.0 | 0.98 |

| Compound | EC50 (μM) | |
|---|---|---|
| DPPH | .O2– | |
| 1 | 20.3 ± 0.2 | 123.4 ± 0.4 |
| 2 | 20.2 ± 0.3 | 83.3 ± 1.2 |
| 3 | 14.1 ± 0.2 | 12.3 ± 0.1 |
| 4 | 9.5 ± 0.2 | 7.0 ± 0.0 |
| 5 | 17.5 ± 0.2 | 23.9 ± 0.3 |
| 6 | 13.6 ± 0.1 | 84.7 ± 0.3 |
| 7 | 15.8 ± 0.2 | 91.1 ± 0.3 |
| 8 | 15.4 ± 0.1 | 74.2 ± 0.4 |
| 9 | 9.0 ± 0.0 | 26.8 ± 0.1 |
| 10 | 7.6 ± 0.0 | 24.0 ± 0.1 |
| Trolox | 43.6 ± 0.9 | 64.1 ± 0.2 |
Experimental
Reagents
Cell culture
Determination of cytotoxicity
Assay for DNA fragmentation
Determination of DPPH radical scavenging activity
Determination of superoxide anion scavenging activity
Statistical analysis
Acknowledgements
References and Notes
- Harborne, J.B. The Flavonoids: Advances in Research from 1986; Champion & Hall: London, UK, 1993. [Google Scholar]
- Harborne, J. B.; Baxter, H. The Handbook of Natural Flavonoids; Wiley: New York, USA, 1999. [Google Scholar]
- Knekt, P.; Jarvinen, R.; Seppanen, R.; Hellovaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef]
- Keli, S.O.; Hertog, M.G.; Feskens E, J.; Kromhout, D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch. Intern. Med. 1996, 156, 637–642. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C.; Feskens, E.J.; Bueno H.B. Mesquita, d.; Kromhout, D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am. J. Clin. Nutr. 2001, 74, 227–232. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors 2006, 27, 19–35. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties, Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar]
- Smith, D.M.; Dou, Q.P. Green tea polyphenol epigallocatechin inhibits DNA replication and consequently induces leukemia cell apoptosis. Int. J. Mol. Med. 2001, 7, 645–652. [Google Scholar]
- Baatout, S.; Jacquet, P.; Derradji, H.; Ooms, D.; Michaux, A.; Mergeay, M. Study of the combined effect of X-irradiation and epigallocatechin-gallate (a tea component) on the growth inhibition and induction of apoptosis in human cancer cell lines. Oncol. Rep. 2004, 12, 159–167. [Google Scholar]
- Vaux, D. L. Toward an understanding of the molecular mechanisms of physiological cell death. Proc. Natl. Acad. Sci. U. S. A. 1993, 90, 786–789. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.H.; Zhang, J.; Yu, B.Y. Comparative Evaluation of Cytotoxicity and Antioxidative Activity of 20 Flavonoids. J. Agric. Food Chem. 1998, 56, 230–235. [Google Scholar]
- Shimomura, O.; Wu, C.; Murai, A.; Nakamura, H. Evaluation of Five Imidazopyrazinone-Type Chemiluminescent Superoxide Probes and Their Application to the Measurement of Superoxide Anion Generated by Listeria monocytogenes. Anal. Biochem. 1998, 258, 230–235. [Google Scholar] [CrossRef]
- Saito, A.; Tanaka, A.; Ubukata, M.; Nakajima, N. Stereoselection of 3,4-cis and 3,4-trans catechin and catechin condensation under intramolecular coupling method. Synlett 2004, 11, 2040–2042. [Google Scholar]
- Saito, A.; Doi, Y.; Tanaka, A.; Matsuura, N.; Ubukata, M.; Nakajima, N. Systematic synthesis of four epicatechin series procyanidin trimers and their inhibitory activity on the Maillard reaction and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 4783–4790. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 2: Stereoselective gram-scale synthesis of procyanidin-B3. Tetrahedron 2002, 58, 7829–7837. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Matsuura, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 5. Highly stereoselective synthesis and inhibitory activity of maillard reaction of 3, 4-trans catechin and epicatechin dimers, procyanidin B1, B2, B3, B4 and their acetates. Heterocycles 2004, 62, 479–489. [Google Scholar]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 3. Stereoselective 3, 4-cis catechin and catechin condensation by TMSOTf-catalyzed intramolecular coupling method. Tetrahedron Lett. 2003, 44, 5449–5452. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 4. The synthesis of procyanidin B1 and B4: TMSOTf-catalyzed cyclization of catechin and epicatechin condensation. Heterocycles 2003, 61, 287–298. [Google Scholar] [CrossRef]
- Saito, A.; Tanaka, A.; Ubukata, M.; Nakajima, N. Efficient stereoselective synthesis of proanthocyanidin trimers with TMSOTf-catalyzed intermolecular condensation. Synlett. 2004, 6, 1069–1073. [Google Scholar]
- Sample Availability: Contact the authors to request samples of polyphenols 6-10.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mitsuhashi, S.; Saito, A.; Nakajima, N.; Shima, H.; Ubukata, M. Pyrogallol Structure in Polyphenols is Involved in Apoptosis-induction on HEK293T and K562 Cells. Molecules 2008, 13, 2998-3006. https://doi.org/10.3390/molecules13122998
Mitsuhashi S, Saito A, Nakajima N, Shima H, Ubukata M. Pyrogallol Structure in Polyphenols is Involved in Apoptosis-induction on HEK293T and K562 Cells. Molecules. 2008; 13(12):2998-3006. https://doi.org/10.3390/molecules13122998
Chicago/Turabian StyleMitsuhashi, Shinya, Akiko Saito, Noriyuki Nakajima, Hiroshi Shima, and Makoto Ubukata. 2008. "Pyrogallol Structure in Polyphenols is Involved in Apoptosis-induction on HEK293T and K562 Cells" Molecules 13, no. 12: 2998-3006. https://doi.org/10.3390/molecules13122998




