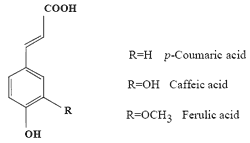
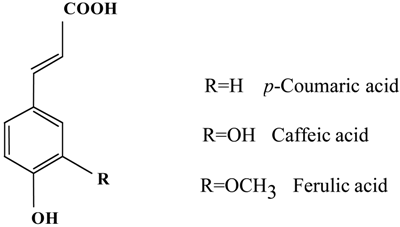
A Comparative Study of the Radical-scavenging Activity of the Phenolcarboxylic Acids Caffeic Acid, p-Coumaric Acid, Chlorogenic Acid and Ferulic Acid, With or Without 2-Mercaptoethanol, a Thiol, Using the Induction Period Method
Abstract
:Introduction
Results and Discussion
Radical scavenging activities determined by the induction period method
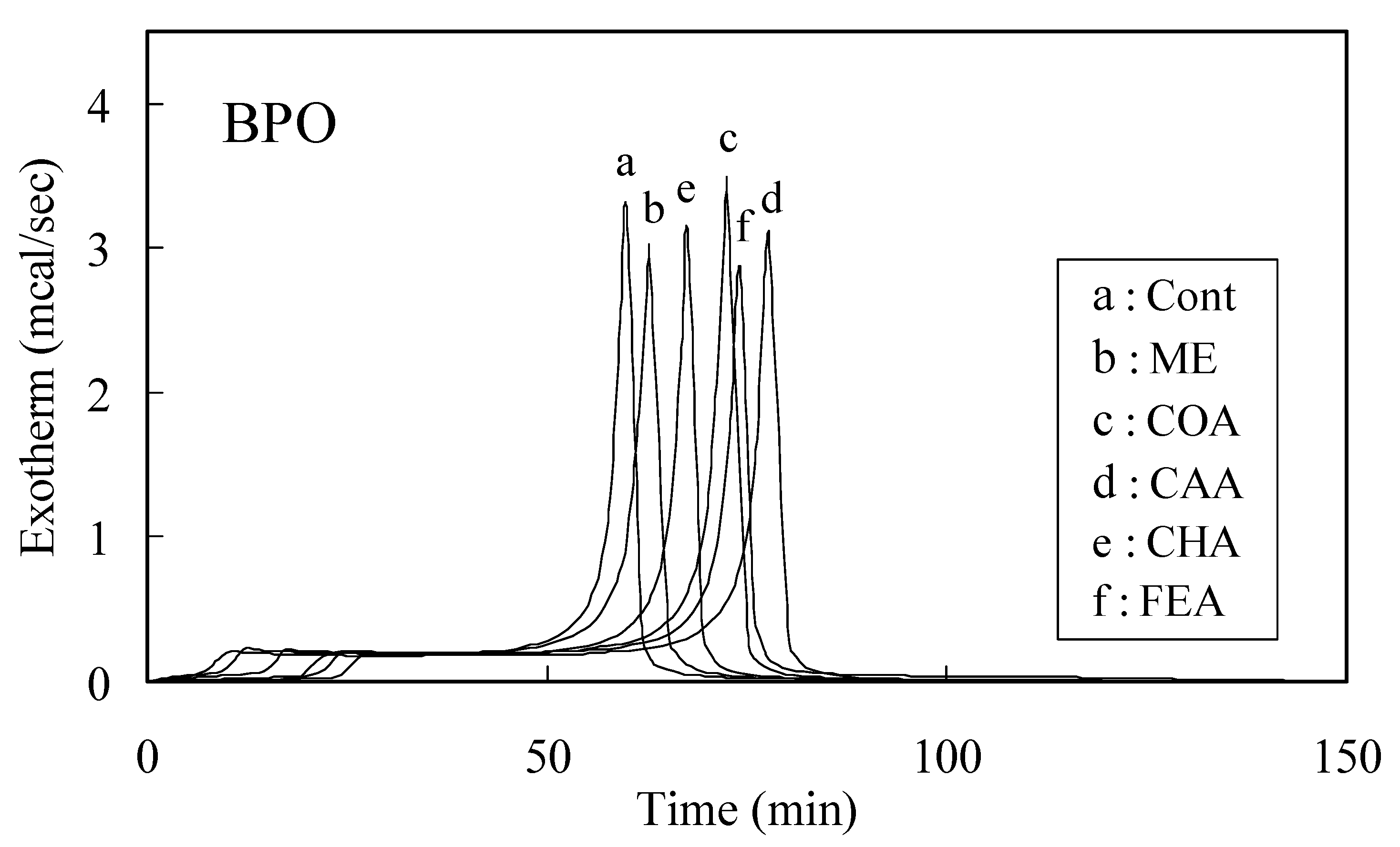
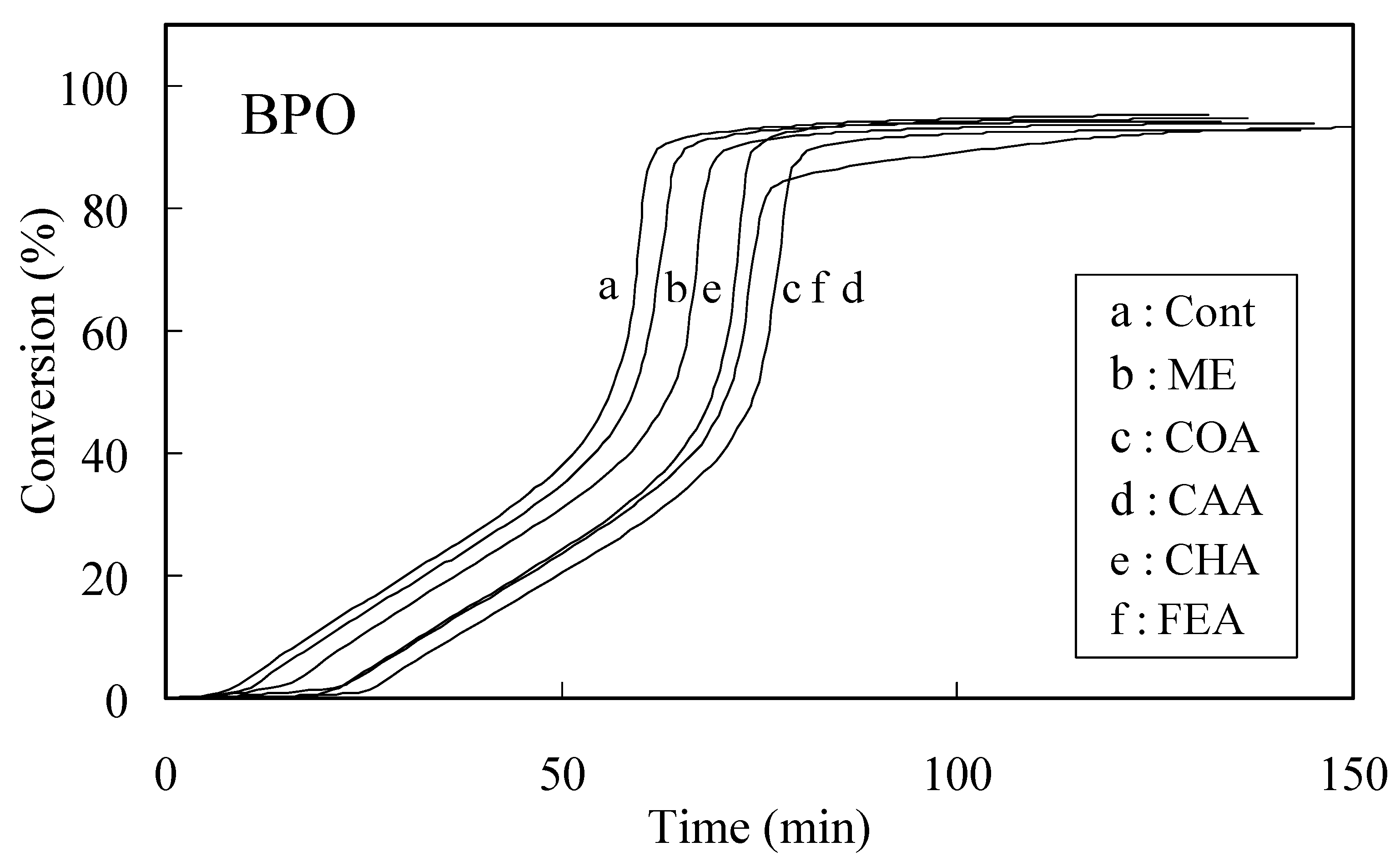
| Caffeic acid | p-Coumaric acid | Chlorogenic acid | Ferulic acid | |
|---|---|---|---|---|
| AIBN | 20.22 | 26.47 | 32.63 | 20.65 |
| BPO | 7.18 | 9.60 | 17.57 | 9.21 |
| Initiator | Phenolsa | IP (min) | |||
|---|---|---|---|---|---|
| observed (A) | calculated (B) | B-A | A/B | ||
| AIBN | Caffeic acid | 3.828 | |||
| AIBN | Caffeic acid +ME | 4.893 | 4.135 | -0.758 | 1.183** |
| AIBN | Chlorogenic acid | 2.348 | |||
| AIBN | Chlorogenic acid + ME | 2.694 | 2.655 | -0.039 | 1.014 |
| AIBN | p-Coumaric acid | 2.966 | |||
| AIBN | p-Coumaric acid + ME | 3.658 | 3.336 | -0.322 | 1.097* |
| AIBN | Ferulic acid | 3.757 | |||
| AIBN | Ferulic acid + ME | 4.137 | 4.064 | -0.073 | 1.018 |
| AIBN | ME | 0.307 | |||
| BPO | Caffeic acid | 17.234 | |||
| BPO | Caffeic acid +ME | 17.571 | 19.523 | 1.952 | 0.9* |
| BPO | Chlorogenic acid | 6.371 | |||
| BPO | Chlorogenic acid + ME | 7.887 | 8.236 | 0.349 | 0.958 |
| BPO | p-Coumaric acid | 12.841 | |||
| BPO | p-Coumaric acid + ME | 10.867 | 14.77 | 3.903 | 0.736** |
| BPO | Ferulic acid | 13.405 | |||
| BPO | Ferulic acid + ME | 14.928 | 15.334 | 0.406 | 0.974 |
| BPO | ME | 1.929 | |||
Mixtures of ME with caffeic acid, p-coumaric acid, chlorogenic acid or ferulic acid
| Compounds | a Cytotoxicity CC50, microM | Radical-scavenging activity | ||||
|---|---|---|---|---|---|---|
| bNO | O2- | .OH | eBDE | fE1/2 | ||
| EC50, microM | cSOD U mg-1 | dEC50, mM | kcal/mol | mV | ||
| Caffeic acid | 13 | 0.5 | 305 | 0.77 | 70.8, 73.1* | 531 |
| p-Coumaric acid | >61 | 17 | <0.1 | 1.07 | 75.2 | 942 |
| Ferulic acid | >52 | 8.3 | <0.1 | 3.22 | 73.10 | 753 |
Conclusions
Experimental
General
DSC measurements
Rate of initiation
Measurement of stoichiometric factor (n)
Measurement of the inhibition rate constant (kinh)
References and Notes
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Jiang, R.W.; Lau, K.M.; Hon, P.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Toda, S. Inhibitory effects of phenylpropanoid metabolites on copper-induced protein oxidative modification of mice brain homogenate, in vitro. Biol. Trace Elem. Res. 2002, 85, 183–188. [Google Scholar] [CrossRef]
- Leenen, R.; Roodenburg, A.J.; Vissers, M.N.; Schuurbiers, J.A.; van Putte, K.P.; Wiseman, S.A.; van de Put, F.H. Supplementation of plasma with olive oil phenols and extracts: influence on LDL oxidation. J. Agric. Food Chem. 2002, 50, 1290–1297. [Google Scholar] [CrossRef]
- Zhang, J.; Stanley, R.A.; Melton, L.D.; Skinner, M.A. Inhibition of lipid oxidation by phenolic antioxidants in relation to their physicochemical properties. Pharmacologyonline 2007, 1, 180–189. [Google Scholar]
- Roche, M.; Dufour, C.; Mora, N.; Dangles, O. Antioxidant activity of olive phenols: mechanistic investigation and characterization of oxidation products by mass spectrometry. Org. Biomol. Chem. 2005, 3, 423–430. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Ogiwara, T.; Satoh, K.; Negoro, T.; Okayasu, H.; Sakagami, H.; Fujisawa, S. Inhibition of NO production by activated macrophages by phenolcarboxylic acid monomers and polymers with radical scavenging activity. Anticancer Res. 2003, 23, 1317–1323. [Google Scholar]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002, 22, 2711–2718. [Google Scholar]
- Kadoma, Y.; Atsumi, T.; Okada, N.; Ishihara, M.; Yokoe, I.; Fujisawa, S. Radical-scavenging activity of natural methoxyphenol vs. synthetic ones using the induction period method. Molecules 2007, 12, 130–138. [Google Scholar] [CrossRef]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O'Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef]
- Amorati, R.; Ferroni, F.; Lucarini, M.; Pedulli, G.F.; Valgimigli, L. Aquantitative approach to the recycling of a-tocopherol by coantioxidants. J. Org. Chem. 2002, 67, 9295–9303. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.N.; Cuvelier, M.E.; Berset, C. Antioxidant activity of phenolic compounds in 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidation: synergistic and antagonistic effects. J. Am. Oil Chem. Soc. 2003, 80, 1007–1012. [Google Scholar] [CrossRef]
- Arakawa, R.; Yamaguch, M.; Hotta, H.; Osakai, T.; Kimoto, T. Product analysis of caffeic acid oxidation by on-line electrochemistry/electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1228–1236. [Google Scholar] [CrossRef]
- Cilliers, J.J.L.; Singleton, V.L. Characterization of the products of nonenzymic autoxidative phenolic reactions in a caffeic acid model system. J. Agr. Food Chem. 1991, 39, 1298–1303. [Google Scholar] [CrossRef]
- Moane, S.; Park, S.; Lunte, C.E.; Smyth, M.R. Detection of phenolic acids in beverages by capillary electrophoresis with electrochemical detection. Analyst 1998, 123, 1931–1936. [Google Scholar] [CrossRef]
- Selassie, C.D.; Shusterman, A.J.; Kapur, S.; Verma, R.P.; Zhang, L.; Hansch, C. On the toxicity of phenols to fast growing cells. A QSAR model for a radical-based toxicity. J. Chem. Soc., Perkin Trans. 2 1999, 2729–2733. [Google Scholar]
- Henríquez, C.; Bueno, C.; Lissi, E.A.; Encinas, M.V. Thiols as chain transfer agents in free radical polymerization in aqueous solution. Polymer 2003, 44, 5559–5561. [Google Scholar] [CrossRef]
- Li, Y.; Trush, M.A. Reactive oxygen-dependent DNA damage resulting from the oxidation of phenolic compounds by a copper-redox cycle mechanism. Cancer Res. (Suppl.) 1994, 54, 1895s–1898s. [Google Scholar]
- Moridani, M.Y.; Scobie, H.; Jamshidzadeh, A.; Salehi, P.; O'Brien, P.J. Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: glutathione conjugate formation. Drug Metab. Dispos. 2001, 29, 1432–1439. [Google Scholar]
- Sample Availability: Available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kadoma, Y.; Fujisawa, S. A Comparative Study of the Radical-scavenging Activity of the Phenolcarboxylic Acids Caffeic Acid, p-Coumaric Acid, Chlorogenic Acid and Ferulic Acid, With or Without 2-Mercaptoethanol, a Thiol, Using the Induction Period Method. Molecules 2008, 13, 2488-2499. https://doi.org/10.3390/molecules13102488
Kadoma Y, Fujisawa S. A Comparative Study of the Radical-scavenging Activity of the Phenolcarboxylic Acids Caffeic Acid, p-Coumaric Acid, Chlorogenic Acid and Ferulic Acid, With or Without 2-Mercaptoethanol, a Thiol, Using the Induction Period Method. Molecules. 2008; 13(10):2488-2499. https://doi.org/10.3390/molecules13102488
Chicago/Turabian StyleKadoma, Yoshinori, and Seiichiro Fujisawa. 2008. "A Comparative Study of the Radical-scavenging Activity of the Phenolcarboxylic Acids Caffeic Acid, p-Coumaric Acid, Chlorogenic Acid and Ferulic Acid, With or Without 2-Mercaptoethanol, a Thiol, Using the Induction Period Method" Molecules 13, no. 10: 2488-2499. https://doi.org/10.3390/molecules13102488