An Improved Synthesis of 4-Chlorocoumarin-3-sulfonyl Chloride and Its Reactions with Different Bidentate Nucleophiles to Give Pyrido[1',2':2,3]- and Thiazino[3',2':2,3]-1,2,4-Thiadiazino[6,5-c]Benzopyran-6-one 7,7-Dioxides
Abstract
:Introduction
Results and Discussion


Experimental
General
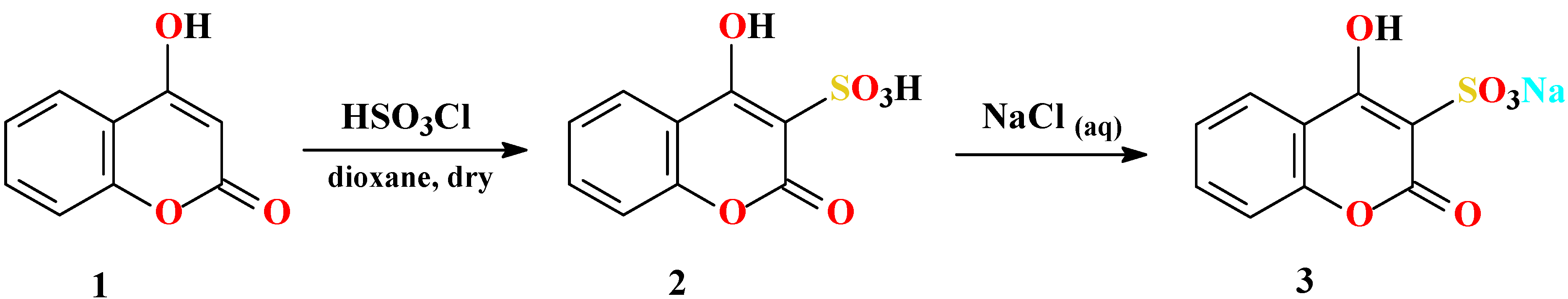
Synthesis of 4-hydroxycoumarin-3-sulfonic acid (2)
Synthesis of sodium 4-hydroxycoumarin-3-sulfonate (3)
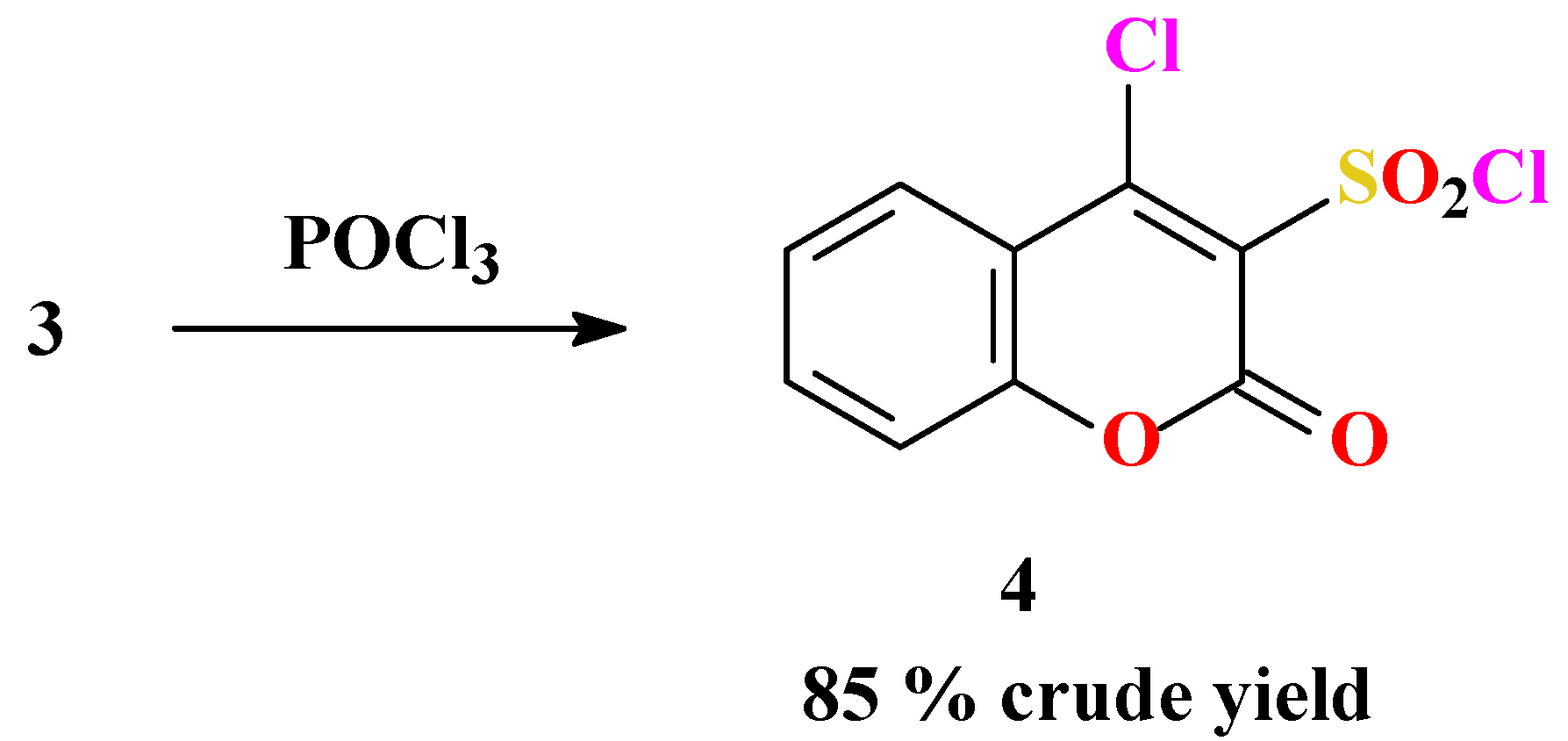
Synthesis of 4-chlorocoumarin-3-sulfonyl chloride (4)
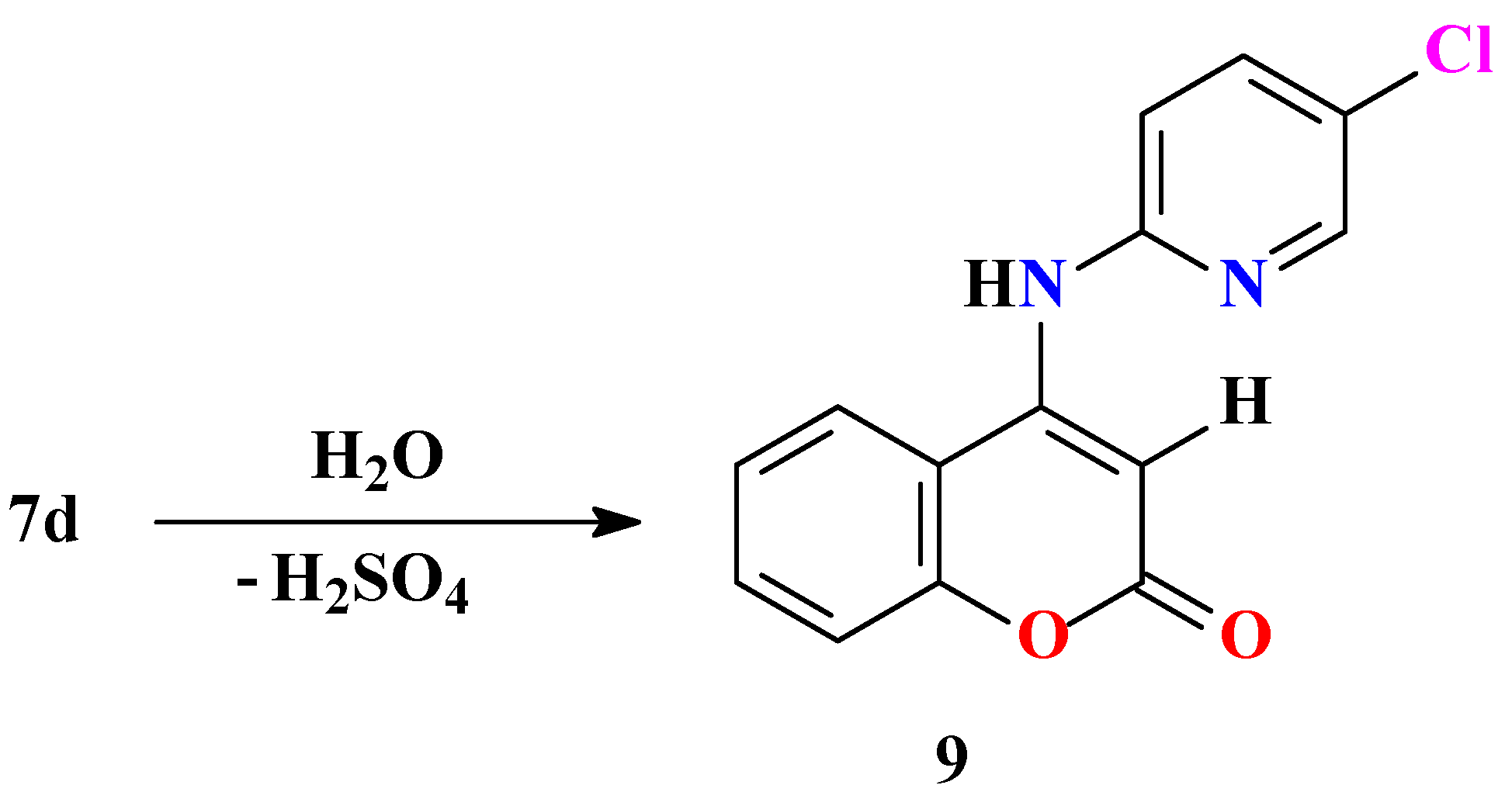
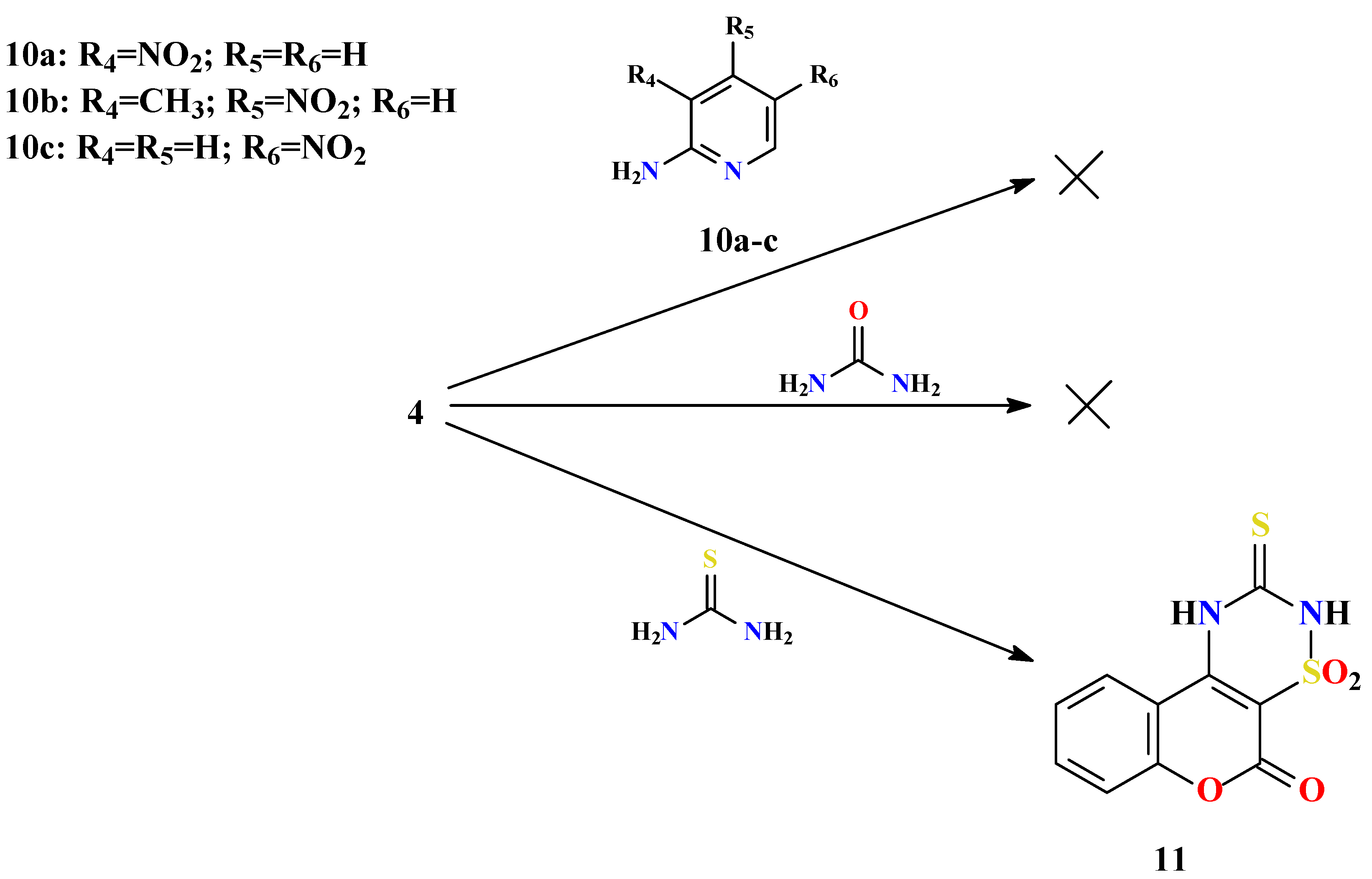
General procedure for preparation of substitutedpyrido[1’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzo-pyran-6-one 7,7-dioxides 7a-f and thiazolo[3’,2’:2,3]-1,2,4-thiadiazino[6,5-c]benzopyran-6-one 7,7-dioxides 8a,b
Acknowledgements
References and Notes
- Gupta, R. R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry; Springer-Verlag: Berlin, 1998. [Google Scholar]
- Fed. Reg. 19 1239 (Mar. 5, 1954)
- Sax, N. I. Dangerous Properties of Industrial Materials (4th Ed.); Van Nostrand Co.: New York, 1975. [Google Scholar]
- Manolov, I.; Danchev, N.D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarins. Eur. J. Med. Chem. Chim. Ther. 1995, 30, 531–536. [Google Scholar] [CrossRef]
- Arora, R. B.; Mathur, C. N. Relationship between structure and anticoagulant activity of coumarin derivatives. Brit. J. Pharmacol. 1963, 20, 29–35. [Google Scholar]
- Ziegler, E.; Rossmann, U. Chemistry of 4-hydroxycoumarins. VIII. Synthesis of anticoagulants. Monatsh. Chem. 1957, 88, 25–34. [Google Scholar] [CrossRef]
- Al-Haiza, M. A.; Mostafa, M. S.; El-Kady, M. Y. Synthesis and Biological Evaluation of Some New Coumarin Derivatives. Molecules 2003, 8, 275–286. [Google Scholar]
- Dutton, C. J.; Sutcliffe, J.; Yang, B. 4-Hydroxy coumarin derivatives with antibacterial activity. PCT Int. Appl. US1993/006308 1994. [Google Scholar]
- Parmar, V. S.; Bisht, K. S.; Jain, R.; Singh, S.; Sharma, S. K.; Gupta, S.; Malhotra, S.; Tyagi, O. D.; Vardhan, A.; Pati, H. N.; Berghe, D. V.; Vlietinck, A. J. Synthesis, Antimicrobial and Antiviral Activities of Novel Polyphenolic Compounds. Ind. J. Chem. Sec. B 1996, 35, 220–232. [Google Scholar]
- Ishikawa, T.; Kotake, K.I.; Ishii, H. Synthesis of Toddacoumaquinone, a Coumarin-Naphtho-quinone Dimer, and Its Antiviral Activities. Chem. Pharm. Bull. 1995, 43, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Nofal, Z. M.; El-Zahar, M. I.; Abd El-Karim, S. S. Novel Coumarin Derivatives with Expected Biological Activity. Molecules 2000, 5, 99–113. [Google Scholar]
- Raev, L.; Voinova, E.; Ivanov, I.; Popov, D. Antitumor activity of some coumarin derivatives. Pharmazie 1990, 45, 696–702, [Chem. Abstr. 1990, 114, 74711 B]. [Google Scholar]
- Valenti, P.; Rampa, A.; Recanatini, M.; Bisi, A.; Belluti, F.; Da Re, P.; Carrara, M.; Cima, L. Synthesis, cytotoxicity and SAR of simple geiparvarin analogues. Anticancer Drug Des. 1997, 12, 443–451. [Google Scholar] [PubMed]
- Shah, A.; Naliapara, Y.; Sureja, D.; Motohashi, N.; Kawase, M.; Miskolci, C.; Szabo, D.; Molnar, J. 6,12-Dihydro-1-Benzopyrano[3,4-b][1,4]Benzothiazin-6-ones Synthesis and MDR Reversal in Tumor Cells. Anticancer Res. 1998, 18, 3001–3004. [Google Scholar]
- El-Sayed, A. M.; Abd-Allah, O. A. Synthetic and Biological Studies on Coumarin Hydrazone Derivatives. Phosporus, Sulfur Silicon Relat. Elem. 2001, 170, 75–86. [Google Scholar]
- El-Agrody, A. M.; Abd El-Latif, M. S.; El-Hady, N. A.; Fakery, A. H.; Bedair, A. H. Hetero-aromatization with 4-hydroxycoumarin. Part II. Molecules 2001, 6, 519–527. [Google Scholar]
- Emmanuel-Giota, A. A.; Fylaktakidou, K. C.; Hadjipavlou-Litina, D. J.; Litinas, K. E.; Nicolaides, D. N. Synthesis and biological evaluation of several 3-(coumarin-4-yl)-tetrahydroisoxazole and 3-(coumarin-4-yl)-dihydropyrazole derivatives. J. Heterocycl. Chem. 2001, 38, 717–722. [Google Scholar]
- Spino, C.; Dodier, M.; Sotheeswaran, S. Anti-HIV Coumarins from Calophyllum Seed Oil. Bioorg. Med. Chem. Lett. 1998, 8, 3475–3478. [Google Scholar] [CrossRef] [PubMed]
- Thaisrivongs, S.; Watenpaugh, K. D.; Howe, W. J.; Tomich, P. K.; Dolak, L. A.; Chong, K.-T.; Tomich, C.-S. C.; Tomasselli, A. G.; Turner, S. R.; Strohbach, J. W.; Mulichak, A. M.; Janakiraman, M. N.; Moon, J. B.; Lynn, J. C.; Horng, M.-M.; Hinshaw, R. R.; Curry, K. A.; Rothrock, D. J. Structure-Based Design of Novel HIV Protease Inhibitors: Carboxamide-Containing 4-Hydroxycoumarins and 4-Hydroxy-2-pyrones as Potent Nonpeptidic Inhibitors. J. Med. Chem. 1995, 38, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.; Khilevich, A.; Filer., C.; Rizzo, J. D.; Giltner, J.; Flavin, M. T.; Xu, Z. Q. Synthesis of Dual C-14-Labeled (+)-Calanolide-A, a Naturally-Occurring Anti-HIV Agent. J. Label. Comp. Radiopharm. 1997, 39, 901–906. [Google Scholar]
- Zhao, H.; Neamati, N.; Hong, H.; Mazumder, A.; Wang, S.; Sunder, S.; Milner, G. W. A.; Pommier, Y.; Burke, T.R., Jr. Coumarin-Based Inhibitors of HIV Integrase. J. Med. Chem. 1997, 40, 242–249. [Google Scholar] [CrossRef] [PubMed]
- García-Valverde, M.; Tomás, T. Sulfur-Nitrogen Heterocycles. Molecules 2005, 10, 318–320. [Google Scholar]
- Vega, S.; Diaz, J. A.; Arranz, E. Thieno- and pyrazolo-1,2,4-thiadiazino-1,1-dioxides with anti-HIV activity. ES2136013 1999. [Google Scholar]
- Arranz, M. E.; Diaz, J. A.; Ingate, S. T.; Witvrouw, M.; Pannecouque, C.; Balzarini, J.; De Clerq, E.; Vega, S. Synthesis and anti-HIV activity of 1,1,3-trioxo-2H,4H-thieno[3,4-e][1,2,4]-thiadiazines (TTDs): a new family of HIV-1 specific non-nucleozide reversetranscriptase inhibitors. Bioorg. Med. Chem. 1999, 7, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.; Arranz, M. E. 4H-Thieno[3,4-e]- and 4H-pyrazolo[4,3-e]-1,2,4-thiadiazine 1,1-dioxides. Synthesis, chemical properties and evaluation of their potential cardiovascular activity. J. Heterocycl. Chem. 2004, 41, 45–50. [Google Scholar]
- Di Bella, M.; Monzani, A.; Andrisano, M. G.; Fabio, U.; Quaglio, G. P. Antimicrobial effect of derivatives of 1,2,4-benzothiadiazine-1,1-dioxide. Part VII. Ediz. Scient. 1979, 34, 81–88. [Google Scholar]
- Robertson, D. W.; Steinberg, M. I. Potassium channel modulators: scientific applications and therapeutic promise. J. Med. Chem. 1990, 33, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Longman, S. D; Hamilton, T. C. Potassium channel activator drugs: mechanism of action, pharmacological properties, and therapeutical potential. Med. Res. Rev. 1992, 12, 73–148. [Google Scholar]
- Tabakovic, K.; Tabakovic, I.; Trkovnik, M.; Trinajstic, N. Chemistry of Coumarin.-Nucleophilic Substitutions of 4-Chloro-3-nitrocoumarin with Hard and Soft Nucleophiles. Liebigs Ann. Chem. 1983, 11, 1901–1909. [Google Scholar]
- Checchi, S.; Pecori, V. L.; Bambagiotti, A. M. 4-Hydroxycoumarins. VII. Reactivity of 4-chloro- and 4-hydroxycoumarin-3-sulfonyl chlorides. Gazz. Chim. Ital. 1967, 97, 1749–1761. [Google Scholar]
- Vogel, A. I. Vogel’s Text-book of Practical Organic Chemistry, 5th ed.; Longman: London, 1989; pp. 873-874; 874-878. [Google Scholar]
- Huebner, C. F.; Link, K. P. Studies on 4-hydroxycoumarin. VII. Reactions of 4-hydroxycoumarin with cationoid reagents. J. Am. Chem. Soc. 1945, 67, 99–102. [Google Scholar]
- CCDC 646550 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing [email protected], or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033
- Smith, M. B. March’s Advanced Organic Chemistry; Reactions, Mechanism and Structure, 5th ed.; John Wiley & Sons, Inc.: New York, 2001; pp. 363–364. [Google Scholar]
- Kovác, M.; Sabatié, A.; Floch, L. Synthesis of coumarin sulfonamides and sulfonylurea. ARKIVOC 2001, 100–108. [Google Scholar]
- Sample Availability: Samples of compounds 2, 3, 4, 7a-f, 8a,b, 9 and 11 are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jashari, A.; Hey-Hawkins, E.; Mikhova, B.; Draeger, G.; Popovski, E. An Improved Synthesis of 4-Chlorocoumarin-3-sulfonyl Chloride and Its Reactions with Different Bidentate Nucleophiles to Give Pyrido[1',2':2,3]- and Thiazino[3',2':2,3]-1,2,4-Thiadiazino[6,5-c]Benzopyran-6-one 7,7-Dioxides. Molecules 2007, 12, 2017-2028. https://doi.org/10.3390/12082017
Jashari A, Hey-Hawkins E, Mikhova B, Draeger G, Popovski E. An Improved Synthesis of 4-Chlorocoumarin-3-sulfonyl Chloride and Its Reactions with Different Bidentate Nucleophiles to Give Pyrido[1',2':2,3]- and Thiazino[3',2':2,3]-1,2,4-Thiadiazino[6,5-c]Benzopyran-6-one 7,7-Dioxides. Molecules. 2007; 12(8):2017-2028. https://doi.org/10.3390/12082017
Chicago/Turabian StyleJashari, Ahmed, Evamarie Hey-Hawkins, Bozhana Mikhova, Gerald Draeger, and Emil Popovski. 2007. "An Improved Synthesis of 4-Chlorocoumarin-3-sulfonyl Chloride and Its Reactions with Different Bidentate Nucleophiles to Give Pyrido[1',2':2,3]- and Thiazino[3',2':2,3]-1,2,4-Thiadiazino[6,5-c]Benzopyran-6-one 7,7-Dioxides" Molecules 12, no. 8: 2017-2028. https://doi.org/10.3390/12082017



