Experimental
General
All melting points were measured on a Reichert-Jung Kofler melting point apparatus and are uncorrected; Elementary analysis data were determined with a Heraeus CHN-RAPID instrument; 1H-NMR spectra were recorded on a Bruker Avance 300 (300 MHz) or a Bruker Avance 600 (600 MHz) spectrometer. The solvents used were DMSO-d6 or CDCl3, as indicated; chemical shifts δ are reported in ppm units relative to the internal tetramethylsilane (TMS) standard. The coupling constants are given in Hz, and the different signals are expressed using the standard abbreviations (s, singlet; d, doublet; t, triplet; m, mutiplet; etc). Infrared spectra (IR) were recorded as KBr disks on a Shimadzu IR-435 spectrometer. The assignment data are only the maximum value of the absorption peak. All compounds were routinely checked by TLC. Merck silica gel 60 was used for flash column chromatographic purification. Solvents were reagent grade and when necessary, were purified and dried by standard methods. Concentration of the reaction solutions involved the use of a rotary evaporator at reduced pressure.
General Procedure for the Preparation of Compounds 2
To a suspension of 4-amino-3-furyl-5-sulfanyl-1,2,4-triazole 1 (1 mmol) in ethanol (10 mL) was added dropwise at 0°C an aqueous solution of potassium hydroxide (2 mol∙L-1, 1 mmol). To this mixture the appropriate alkyl halide (1.2 mmol) was added dropwise with vigorous stirring, the temperature was allowed to raise to ambient (25°C) and the reaction was continued for 1-2 h. When the reaction was complete (as checked by TLC), water (20 mL) was added, and a white precipitate was formed, which was separated by filtration, washed with water, and recrystallized from the appropriate solvent.
4-Amino-3-(2-furyl)-5-benzylthio-4H-1,2,4-triazole (2a). This compound was obtained as colorless needles by recrystallization from ethanol; yield 73.2%; mp: 201-203°C; 1H-NMR (300 MHz, DMSO-d6) δ: 7.88 (1H, d, J = 1.7 Hz, 5’-H-furan), 7.43-7.20 (6H, m, Ar-H), 6.68 (1H, d, J = 3.8 Hz, 3’-H-furan), 6.11 (2H, s, NH2), 4.41 (2H, s, -CH2-S-); IR (cm-1): 3272, 3172 (υNH), 3127, 3109 (υ=CH), 3064, 3030 (Ar-H), 1648 (m, vC=N), 1602, 1583, 1462, 1431 (υArC=C), 1247 (s, υN-N=C), 696 (m, υC-S-C); Anal. calcd. for C13H12N4OS: C, 57.21; H, 4.39; N, 20.81; Found: C, 57.33; H, 4.41; N, 20.47.
4-Amino-3-(2-furyl)-5-(3-methoxylbenzyl)thio-4H-1,2,4-triazole (2b). This compound was purified by recrystallization from ethanol to give white needles; yield 67.3 %; mp: 175-178°C; 1H-NMR (300 MHz, DMSO-d6) δ: 7.91 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.24-6.82 (5H, m, Ar-H), 6.71 (1H, d, J = 3.5 Hz, 3’-H-furan), 6.14 (2H, s, NH2), 4.40 (2H, s, -CH2-S-), 3.37 (3H, s, OCH3); IR (cm-1): 3280, 3182 (υNH), 3130, 3109 (υ=CH), 3051, 3010 (Ph-H), 2957, 2939 (υCH3), 1641 (m, vC=N), 1608, 1583, 1465, 1433 (υC=C), 1272 (s, υN-N=C), 709 (m, υC-S-C); Anal. calcd. for C14H14N4O2S: C, 55.40; H, 4.75; N, 18.80; Found: C, 55.51; H, 4.67; N, 18.53.
4-Amino-3-(2-furyl)-5-(4-cyanobenzyl)thio-4H-1,2,4-triazole (2c). This compound was purified by recrystallization from ethanol to afford white needles; yield 83%; mp: 235-236°C; 1H-NMR (300 MHz, DMSO-d6) δ: 7.87 (1H, d, J = 1.2 Hz, 5’-H-furan), 7.76 (2H, AB, d, J = 8.7 Hz, 3,5-H2-Ph), 7.62 (2H, AB, d, J = 8.7 Hz, 2,6-H2-Ph), 7.21 (1H, d, J = 3.6 Hz, 3’-H-furan), 6.68 (1H, dd, J1 = 1.2 Hz, J2 = 3.6 Hz, 4’-H-furan), 6.14 (2H, s, NH2), 4.48 (2H, s, SCH2); IR (cm-1): 3378, 3290 (d, υNH, m), 2225 (υCN, s), 1603, 1517, 1463, 1432, 1414 (s), 1250, 1114, 1017 (s), 978, 902, 748 (s), 556; Anal. calcd. for C14H11N5OS: C, 56.55; H, 3.73; N, 23.55; Found: C, 55.98; H, 3.77; N, 23.65.
4-Amino-3-(2-furyl)-5-(ethoxylcarbonyl)methylthio-4H-1,2,4-triazole (2d). This compound was purified by recrystallization from ethanol to give white needles; yield 71%; mp: 156-158°C; 1H-NMR (300 MHz, DMSO-d6) δ: 7.88 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.21 (1H, d, J = 3.6 Hz, 3’-H-furan), 6.69 (1H, dd, J1 = 1.5 Hz, J2 = 3.6 Hz, 4’-H-furan), 4.44 (2H, s, -CH2-S- ), 4.10 (2H, q, J = 7.5 Hz, OCH2), 1.18 (3H, t, J = 7.5 Hz, CH3); IR (cm-1): 3322 (υNH), 3154, 3128 (υ=CH), 2993, 2972 (υCH3), 1739 (s, υc=o), 1629, 1415, 1380, 1304, 1176 (s), 1024, 997, 898; Anal. calcd. for C10H12N4O3S: C, 44.77; H, 4.51; N, 20.88. Found: C, 44.82; H, 4.47; N, 20.96.
General Procedure for the Preparation of Compounds 3
A mixture of 4-amino-3-furyl-5-sulfanyl-1,2,4-triazole 1 (0.1 mmol) with an aromatic aldehyde in ethanol/water (2:1, 40 mL), was refluxed for about 3 h (checked by TLC). When the reaction solution had cooled down, the crude product was precipitated, collected by filtration and recrystallized from an appropriate solvent.
4-(5-Nitrofurylidene)amino-3-(2-furyl)-5-sulfanyl-4H-1,2,4-triazole (3a). This compound was prepared by the condensation of compound 1 with 5-nitro-2-furaldehyde diacetate in sulfuric acid- ethanol-water (0.1:1:2) solution, and purified by recrystallization from acetone/ethanol (4:1) to give yellow needles; yield 98.3%; mp: 198-201°C; 1H-NMR (300 MHz, DMSO-d6) δ: 14.39 (1H, s, NH or SH), 10.34 (1H, s, -N=CH), 8.01 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.88 (1H, AB, d, J1 = 3.5 Hz, 4’’-H-furan), 7.75 (1H, AB, d, J2 = 3.5 Hz, 3’’-H-furan), 7.28 (1H, d, J = 3.0 Hz, 3’-H-furan), 6.78 (1H, dd, J1 = 1.5 Hz, J2 = 3.0 Hz, 4’-H-furan); IR (cm-1): 3109 (s, υ=CH), 2977, 2936 (w, υCH), 1623 (s, υC=N), 1536 (s, υasNO2), 1450 (s, υC=C), 1349 (υNO2, s), 1274 (υN-N=C, s), 968 (s), 721 (υC-S-C, m); Anal. calcd. for C11H7N5O4S: C, 43.28; H, 2.31; N, 22.94. Found: C, 43.35; H, 2.33; N, 23.05.
4-(4-Methoxybenzylidene)amino-3-(2-furyl)-5-sulfanyl-4H-1,2,4-triazole (3b). This compound was prepared by the reaction of compound 1 with 4-methoxyphenyl aldehyde and purified by recrystalization from ethanol to obtain white needles; yield 72%; mp: 216-218°C; 1H-NMR (600 MHz, DMSO-d6) δ: 12.84 (1H, s, NH or SH), 10.34 (1H, s, -N=CH), 7.66 (1H, d, J = 1.7 Hz, 5’-H-furan), 7.45 (2H, d, J = 6.7 Hz, 2,6-H2-Ph), 7.43 (2H, d, J = 6.7 Hz, 3,5-H2-Ph), 7.20 (1H, d, J = 3.7 Hz, 3’-H-furan), 6.65 (1H, dd, J1 = 1.7 Hz, J2 = 3.7 Hz, 4’-H-furan), 3.99 (3H, s, OCH3); IR (cm-1, KBr): 3101 (m, υAr-H), 2974, 2932 (υ=C-H), 1629, 1569 (υC=N), 1453 (υC-O); Anal. calcd. for: C14H12N4O2S: C, 55.99; H, 4.03; N, 18.65. Found: C, 55.82; H, 4.04; N, 18.56.
4-(3,4-Dimethoxylphenylidene)amino-3-(2-furyl)-5-sulfanyl-4H-1,2,4-triazole (3c). This compound was prepared by the reaction of compound 1 with 3,4-dimethoxylphenyl aldehyde and purified by recrystallization from ethanol to give white needles; yield 56.1%; mp: 225-227°C; 1H-NMR (600 MHz, DMSO-d6) δ: 12.26 (1H, s, NH or SH), 9.88 (1H, s, -N=CH), 7.64 (1H, d, J = 1.8 Hz, 5’-H-furan), 7.56-7.20 (3H, m, Ph-H), 7.01 (1H, d, J = 8.1 Hz, 3’-H-furan), 6.58 (1H, dd, J1 = 1.8 Hz, J2 = 8.1 Hz, 4’-H-furan), 4.00 (3H, s, OCH3), 3.97 (3H, s, OCH3); IR (cm–1): 3137, 3115 (m, νAr-H), 2963, 2933 (νCH3), 1604 (νC=C), 1275 (m, νN-N=C); Anal. calcd. for C15H14N4O3S: C, 54.54; H, 4.27; N, 16.96. Found: C, 54.27; H, 4.13; N, 17.03.
4-(4-Chlorobenzylidene)amino-3-(2-furyl)-5-sulfanyl-4H-1,2,4-triazole (3d). This compound was prepared by the reaction of compound 1 with 4-chlorophenyl aldehyde, purified by recrystallization from ethanol, and obtained as light yellow needles, yield 77%, mp: 226-228°C; 1H-NMR (600 MHz, DMSO-d6) δ: 11.16 (1H, s, NH or SH), 10.40 (1H, s, -N=CH), 7.68 (1H, d, J = 1.3 Hz, 5’-H-furan), 7.52 (2H, d, J = 7.1 Hz, 2,6-H2-Ph), 7.46 (2H, d, J = 7.1 Hz, 3,5-H2-Ph), 6.99 (1H, d, J = 2.9 Hz, 3’-H-furan), 6.58 (1H, dd, J1 = 1.3 Hz, J2 = 2.9 Hz, 4’-H-furan); IR (cm–1): 3081 (m, υAr-H), 2963, 2933 (υ=C-H), 1626, 1562, 1594 (υC=N, C=C); Anal. calcd. for C13H9ClN4OS: C, 51.23; H, 2.98; N, 18.38. Found: C, 51.14; H, 2.96; N, 18.42.
General Procedure for the Preparation of Compounds 4
To a suspension of 4-(arylidene)amino-3-furyl-5-sulfanyl-1,2,4-triazole 3 (1 mmol) in ethanol (10 mL) in ice bath was added dropwise an aqueous solution of KOH (2 mol∙L-1, 1 mmol) with vigorous stirring. When the solid was dissolved, an alkyl halide (1.2 mmol) was added dropwise, and the temperature was raised to ambient (25°C) and kept there for 1-2 h. When the reaction was complete (as indicated by TLC), it was then cooled and a white precipitate was formed, which was separated by filtration, washed with water and recrystallized from a suitable solvent.
4-(5-Nitrofurylidene)amino-3-(2-furyl)-5-benzylthio-4H-1,2,4-triazole (4a). This compound was purified by recrystallization from ethanol to obtain yellow needles; yield 66.4%; mp: 159-161°C; 1H-NMR (300 MHz, DMSO-d6) δ: 8.82 (1H, s, -N=CH), 7.93 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.83 (1H, AB, d, J1 = 3.6 Hz, 4’’-H-furan), 7.65 (1H, AB, d, J2 = 3.6 Hz, 3’’-H-furan), 7.34-7.22 (5H, m, H5-Ph), 7.02 (1H, d, J = 3.6 Hz, 3’-H-furan), 6.71 (1H, dd, J1 = 1.5 Hz, J2 = 3.6 Hz, 4’-H-furan), 4.43 (1H, s, SCH2); IR (cm-1): 3123, 3027 (Ar-H, w), 1572, 1495, 1435, 1347 (s), 1275, 1255, 1014, 964, 743, 710; Anal. calcd. for C15H14N4O3S: C, 54.68; H, 3.31; N, 17.71; Found: C, 54.80; H, 3.35; N, 17.85
4-(5-Nitrofurylidene)amino-3-(2-furyl)-5-(3-methoxylbenzyl)thio-4H-1,2,4-triazole (4b). Separated by flash column chromatography using as eluant cyclohexane/ethyl acetate (1:3), and further purified by recrystallization from ethanol /petroleum ether (bp: 90°C) to give yellow needles; yield 49.2%; mp: 77-79°C; 1H-NMR (300 MHz, DMSO-d6) δ: 8.82 (1H, s, =CH), 7.93 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.84 (1H, d, J = 4.0 Hz, 3’’-H-furan), 7.64 (1H, d, J = 4.0 Hz, 4’’-H-furan), 7.16 (1H, m, Ph-H), 7.01 (1H, d, J = 3.6 Hz, 3’-H-furan), 6.87-6.82 (3H, m, Ph-H), 6.70 (1H, dd, J1 = 1.5 Hz, J2 = 3.6 Hz, 4’-H-furan), 4.37 (2H, s, SCH2), 3.65 (3H, s, OCH3); IR (cm–1): 3091 (νAr-H, s), 1532, 1492, 1441, 1349 (s), 1267, 967; Anal. calcd. for C15H14N4O3S: C, 53.52; H, 3.78; N, 16.42; Found: C, 52.82; H, 3.85; N, 17.01.
4-(5-Nitrofurylidene)amino-3-(2-furyl)-5-(4-cynobenzyl)thio-4H-1,2,4-triazole (4c). This compound was purified by recrystallization from ethanol to give yellow needles; yield 78%; mp: 216-218°C; 1H-NMR (300 MHz, DMSO-d6) δ: 8.85 (1H, s, =CH), 7.92 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.84 (1H, d, J = 4.0 Hz, 4’’-H-furan), 7.73 (2H, d, J = 8.2 Hz, 2,6-H2-Ph), 7.65 (1H, d, J = 4.0 Hz, 3’’-H-furan), 7.52 (2H, d, J = 8.2 Hz, 3,5-H2-Ph), 7.01 (1H, d, J = 3.6 Hz, 3’-H-furan), 6.70 (1H, dd, J1 = 1.5 Hz, J2 = 3.6 Hz, 4’-H-furan), 4.48 (1H, s, SCH2); IR (cm–1): 2226 (νCN), 1531, 1436, 1349, 1272, 1021, 965, 810, 754, 738; Anal. calcd. for C19H12N6O4S: C, 54.28; H, 2.88; N, 19.99. Found: C, 54.40; H, 2.95; N, 20.10.
4-(5-Nitro-2-furylmethylidene)amino-3-(2-furyl)-5-(ethoxylcarbonyl)methylthio-4H-1,2,4-triazole (4d). This compound was purified by recrystallization from ethanol to give yellow needles; yield 80%; mp: 139-140°C; 1H-NMR (300 MHz, DMSO-d6) δ: 8.94 (1H, s, =CH), 7.93 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.86 (1H, d, J = 3.6 Hz, 4’’-H-furan), 7.71 (1H, d, J = 3.6 Hz, 3’’-H-furan), 7.06 (1H, d, J = 3.0 Hz, 3’-H-furan), 6.72 (1H, dd, J1 = 1.5 Hz, J2 = 3.0 Hz, 4’-H-furan), 4.08 (2H, q, J = 7.2 Hz, OCH2), 4.07 (2H, s, SCH2), 1.13 (3H, t, J = 7.2 Hz, CH3); IR (cm–1): 1736 (νC=O, s), 1530, 1442, 1349, 1307, 1278, 1188, 1028, 766; Anal. calcd. for C15H13N5O6S: C, 46.04; H, 3.35; N, 17.89; Found: C, 45.87; H, 3.39; N, 18.08.
4-(3,4-Dimethoxylphenylidene)amino-3-(2-furyl)-5-benzylthio-4H-1,2,4-triazole (4e). This compound was separated by flash column chromatography using as eluant hexane/ethyl acetate (3:2) and further purified by recrystallization from ethanol to give white crystals; yield 48.1%; mp: 100-102°C; 1H-NMR (300 MHz, DMSO-d6) δ: 8.62 (1H, s, =CH), 7.89 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.48-7.12 (8H, m, Ar-H), 7.01 (1H, d, J = 3.6 Hz, 3’-H-furan), 6.70 (1H, dd, J1 = 1.5 Hz, J2 = 3.6 Hz, 4’-H-furan), 4.40 (2H, s, SCH2), 3.85 (3H, s, OCH3), 3.82 (3H, s, OCH3); IR (cm-1): 3100 (ν=CH), 3025 (Ph-H), 2957, 2935 (νCH3), 1598, 1576, 1513 (s), 1274 (νN-N=C, s), 1138 (s), 1026, 1020 (s), 784, 704 (νC-S-C); Anal. calcd. for C22H20N4O3S: C, 62.84; H, 4.79; N, 13.32; Found: C, 62.96; H, 4.83; N, 13.28.
4-(3,4-Dimethoxylphenylidene)amino-3-(2-furyl)-5-(ethoxylcarbonyl)methylthio-4H-1,2,4-triazole (4f). This compound was purified by recrystallization from acetone/cyclohexane (1:1) to give white crystals; yield 33.4%; mp: 196-199°C; 1H-NMR (300 MHz, DMSO-d6) δ: 8.62 (1H, s, N=CH), 7.89 (1H, d, J = 1.5 Hz, 5’-H-furan), 7.31 (1H, m, Ph-H), 7.22 (1H, d, J = 3.0 Hz, 3’-H-furan), 7.01-6.86 (2H, m, Ph-H), 6.68 (1H, dd, J1 = 1.5 Hz, J2 = 3.0 Hz, 4’-H-furan), 4.91 (2H, s, SCH2), 4.13 (2H, q, J = 7.2 Hz, OCH2), 3.69 (3H, s, OCH3), 3.57 (3H, s, OCH3), 1.13 (3H, t, J = 7.2 Hz, CH3); IR (cm-1): 3146 (ν=CH, m), 3020 (Ph-H, m), 2955 (νCH3, w), 1740 (νC=O, s), 1517 (s), 1450, 1450, 1414 (m), 1271 (νN-N=C, s), 1032, 1013, 754 (m); Anal. calcd. for C19H20N4O5S: C, 54.80; H, 4.84; N, 13.45. Found: C, 54.65; H, 4.87; N, 13.53.
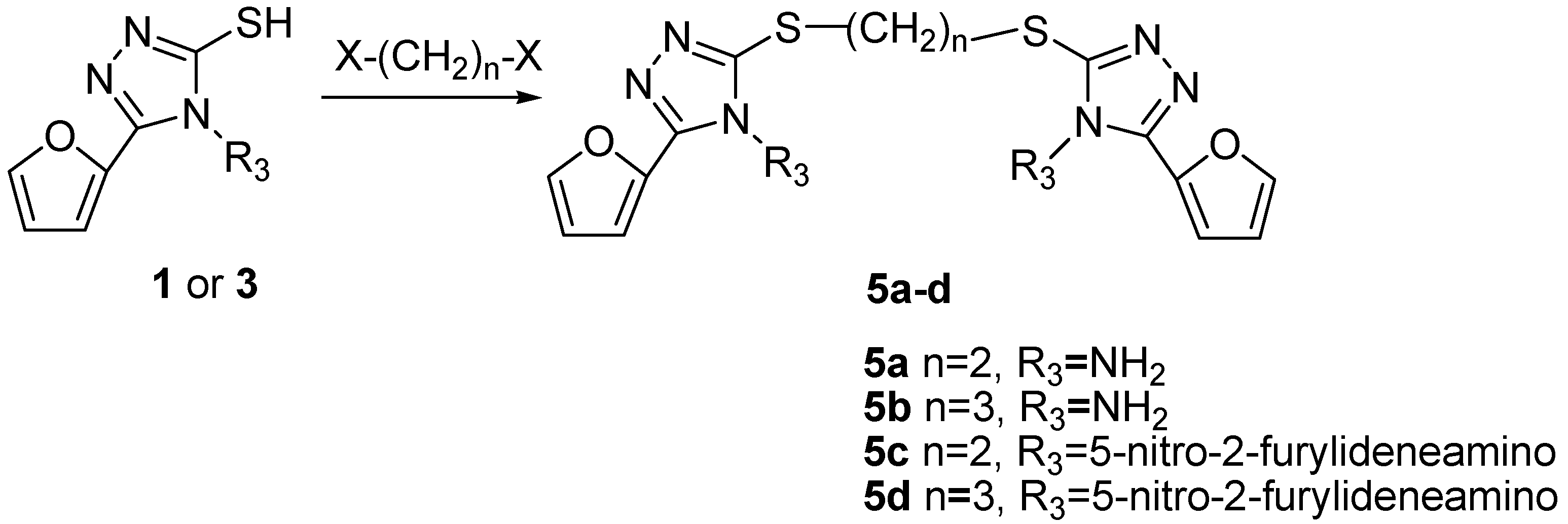
General Procedure for the Preparation of Compounds 5
To a suspension of 4-(arylidene)amino/amino-3-furyl-5-sulfanyl-1,2,4-triazole 1 or 3 (1 mmol) in ethanol (10 mL) in an ice bath was added dropwise an aqueous solution of KOH (2 mol∙L-1, 1 mmol) with vigorous stirring. When the solid was dissolved, the appropriate alkyl dihalide (0.5 mmol) was added dropwise and the temperature was allowed to raise at 25°C for 1 h, then the reaction solution was refluxed for 1-2 h. (checked by TLC). The product was separated by flash column chromatography with suitable eluents and further purified by recrystallization from an appropriate solvent.
1,2-bis[(4-Amino-3-(2-furyl)-4H-1,2,4–triazol)-5-yl]dithioethane (5a). This compound was prepared by the reaction of compound 1 with 1,2-dibromoethane under reflux for 2 days, and purified by recrystallization from ethanol to obtain a yellow solid; yield 52%; mp: 289-291°C; 1H-NMR (600 MHz, DMSO-d6) δ: 7.90 (2H, d, J = 1.5 Hz, 5’-H-furan), 7.24 (2H, d, J = 3.4 Hz, 3’-H- furan), 6.71 (2H, dd, J1 = 1.8 Hz, J2 = 3.4 Hz, 4’-H-furan), 6.15 (4H, s, NH2), 3.55 (4H, t, J = 19.8 Hz); IR (cm-1): 3329, 3258 (m, νN-H), 3181, 3134 (ν=CH), 2977, 2947 (w, νCH2), 1628 (s, νC=N), 1526 (νasNO2), 1432, 1416 (s, νC=C, νN-N=C), 1018, 898, 751 (s), 738 (s); Anal. calcd. for C14H14N8O2S2: C, 43.07; H, 3.61; N, 28.70. Found: C, 43.01; H, 3.63; N, 28.64.
1,3-bis[(4-Amino-3-(2-furyl)-4H-1,2,4-triazol)-5-yl]dithiopropane (5b). This compound was prepared from compound 1 reacted with 1,3-dibromopropane under reflux for 2 day, and purified by recrystallization from ethanol to obtain a yellow solid; yield 62%; mp: 297-299°C; 1H-NMR (600 MHz, DMSO-d6) δ: 7.89 (2H, d, J = 1.8 Hz, 5’-H-furan), 7.23 (2H, d, J = 3.4 Hz, 3’-H-furan), 6.70 (2H, dd, J1 = 1.8 Hz, J2 = 3.4 Hz, 4’-H-furan), 6.13 (4H, s, NH2), 3.29 (4H, t, J = 18.8 Hz, CH2-S), 2.13 (2H, m, CH2); IR (cm-1): 3327, 3284 (m, νN-H), 3174, 3130 (ν=CH), 2977, 2947 (νCH2), 1635, 1432, 1416 (s, νC=C, νN-N=C), 1021, 1009, 756 (s), 740 (s); Anal. calcd. for C15H16N8O2S2: C, 44.54; H, 3.99; N, 27.70; Found: C, 44.56; H, 4.01; N, 27.78.
1,2-bis[(4-(5-Nitro-2-furylmethylidene)amino-3-(2-furyl)-4H-1,2,4-triazol)-5-yl]dithioethane (5c). This compound was prepared from the reaction of compound 3a with dibromoethane, and purified by recrystallization from ethanol to give a yellow solid; yield 61.2%; mp: 220°C; 1H-NMR (600 MHz, DMSO-d6) δ: 8.89 (2H, s, -N=CH), 7.92 (2H, d, J = 1.8 Hz, 5’-H-furan), 7.83 (2H, d, J = 3.9 Hz, 4’’-H-furan), 7.68 (2H, d, J = 3.9 Hz, 3’’-H-furan), 7.02 (2H, d, J = 3.6 Hz, 3’-H-furan), 6.70 (2H, dd, J1 = 1.8 Hz, J2 = 3.6 Hz, 4’-H-furan), 3.54 (4H, s, CH2-S); IR (cm-1): 3113, 3009 (ν=CH), 2923 (νCH), 1568, 1437 (νC=C), 1526 (νasNO2), 1349 (s, νsNO2), 1258 (s, νN-N=C), 713 (νC-S-C); Anal. calcd. for C24H16N10O8S2: C, 45.28; H, 2.53; N, 20.00; Found: C, 45.26; H, 2.60; N, 19.98.
1,3-bis[(4-(5-Nitro-2-furylmethylidene)amino-3-(2-furyl)-4H-1,2,4-triazol)-5-yl]dithiopropane (5d). This compound was prepared from the reaction of compound 3a with dibromopropane, purified by recrystallization from ethanol and obtained as yellow solid; yield 61%; mp: 220°C; 1H-NMR (600 MHz, DMSO-d6) δ: 8.92 (2H, s, -N=CH), 7.93 (2H, d, J = 1.7 Hz, 5’-H-furan), 7.84 (2H, d, J = 4.0 Hz, 4’’-H-furan), 7.70 (2H, d, J = 4.0 Hz, 3’’-H-furan), 7.04 (2H, d, J = 3.4 Hz, 3’-H-furan), 6.72 (2H, dd, J1 = 1.7 Hz, J2 = 3.4 Hz, 4’-H-furan), 3.30 (4H, t, J = 7.0 Hz, CH2-S), 2.1 (2H, m, CH2 ); IR (cm-1): 3150, 3112 (w, ν=CH), 2913 (νCH), 1527, 1437 (νC=C, C=N), 1527 (νasNO2), 1350 (s, νsNO2); Anal. calcd. for C25H18N10O8S2: C, 46.15; H, 2.79; N, 21.53. Found: C, 46.22; H, 2.81; N, 21.56.
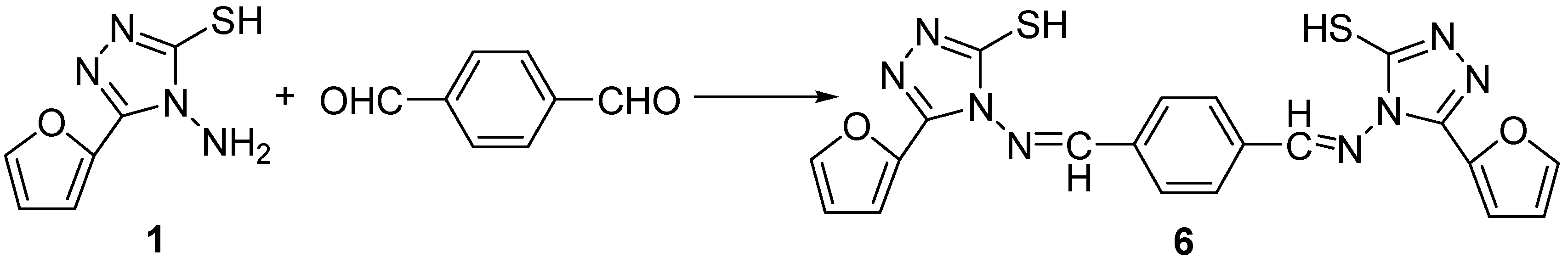
Preparation of 1,4-bis[(4-Iminomethyl-3-(2-furyl)-5-mercapto-4H-1,2,4-triazole -4-yl] phene (6). The preparation of compound 6 by the reaction of compound 3a with 1,4-diformylbenzene in refluxing ethanol for 2 h used the same conditions of compounds 3a-d and the product was obtained as a white solid; yield 61%; mp: 264-265°C; 1H-NMR (600 MHz, DMSO-d6) δ: 14.30 (2H, s, NH or SH), 10.04 (2H, s, -N=CH), 8.17 (4H, s, Ph-H4), 7.99 (2H, d, J = 1.7 Hz, 5’-H-furan), 7.18 (2H, d, J = 3.4 Hz, 3’-H-furan), 6.72 (2H, dd, J1 = 1.7 Hz, J2 = 3.4 Hz, 4’-H-furan); IR (cm-1): 3423 (νNH), 3087, 3015 (m, ν=CH), 2965, 2992, 2923 (w, νCH), 1626 (νC=N), 1527, 1452 (νC=C, C=N), 1266 (s), 969, 749; Anal. calcd. for C20H14N8O2S2: C, 51.94; H, 3.05; N, 24.23. Found: C, 51.87; H, 3.02; N, 24.29.
Preparation of 4-[2-(5-nitro)furylmethylidene]amino-3-(2-furyl)-5-mercapto-piperidylmethyl-4H- 1,2,4-triazole (7a). Compound 3a (10 mmol) was dissolved in a mixed ethanol and dioxane solvent (2:1, 90 mL), then formaldehyde (40%, 1.5 mL) and piperidine (10 mmol) in ethanol (100 mL) were added to this solution. The mixture was stirred for 1-2 h and then kept overnight at room temperature. The solid was collected by filtration and recrystallized from a mixture of ethanol and dioxane (2:1) to yield the title compound 7a; yield: 80%; mp: 195-197°C; 1H-NMR (600 MHz, DMSO-d6) δ: 10.28 (1H, s, N=CH), 8.00 (1H, d, J = 1.7 Hz, 5’-H-furan), 7.84 (1H, d, J = 4.0 Hz, 3’’-H-furan), 7.71 (1H, d, J = 4.0 Hz, 4’’-H-furan), 7.29 (1H, d, J = 3.5 Hz, 3’-H-furan ), 6.78 (1H, dd, J1 = 1.7 Hz, J2 = 3.5 Hz, 4’-H-furan), 5.12 (2H, s, N-CH2-N), 2.74 (4H, t, J = 5.2 Hz, 3,5-H4-piperidine), 1.50 (4H, d, J = 4.7 Hz, 2,6-H4-piperidine), 1.34 (2H, d, J = 5.3 Hz, 4-H2-piperidine); IR (cm-1): 3141, 3120 (w, ν=CH), 2942, 2930 (w, νCH2), 1621, 1530, 1497, 1459, 1434, 1426 (νC=C, C=N), 1349, 1303, 1277, 764; Anal. calcd. for C17H18N6O4S: C, 50.74; H, 4.51; N, 20.88. Found: C, 50.80; H, 4.49; N, 20.9.
Preparation of 4-[2-(5-nitro)furylmethylidene]amino-3-(2-furyl)-5-mercapto-(bis-n-butylamino)-4H-1,2,4-triazole (8). Compound 3a (10 mmol) was dissolved in a mixed ethanol and dioxane solvent (2:1, 90 mL), then formaldehyde (40%, 1.5 mL) and di-n-butylamine (10 mmol) in ethanol (100 mL) were added to this solution. The mixture was refluxed under stirring for 1 h. The white solid formed was collected by filtration and recrystallized from a mixture of ethanol and dioxane (1:1) to give the title compound 8; yield: 78%; mp: 214-215°C; 1H-NMR (600 MHz, DMSO-d6) δ: 11.20 (1H, s, N=CH), 7.84 (1H, d, J = 1.7 Hz, 5’-H-furan), 7.84 (1H, d, J = 4.0 Hz, 3’’-H-furan), 7.83 (1H, d, J = 4.0 Hz, 4’’-H-furan), 7.52 (1H, d, J = 3.5 Hz, 3’-H-furan ), 6.68 (1H, dd, J1 = 1.7 Hz, J2 = 3.5 Hz, 4’-H-furan), 2.81 (4H, t, J = 7.6 Hz, N-(CH2-)2), 1.54 (4H, m, 2CH2), 1.36 (4H, m, 2CH2), 0.92 (6H, t, J = 7.4 Hz, 2CH3); IR (cm-1): 3110 (w, ν=CH), 2962, 2934, 2862 (w, νCH2), 1530, 1494, 1450 (νC=C, C=N), 1374, 1350, 1253; Anal. calcd. for C19H24N6O4: C, 56.99; H, 6.04; N, 20.99. Found: C, 57.12; H, 5.99; N, 21.02.
Anti-HIV-1 assays in MT-4 cells culture
Activity of the compounds against HIV-1 (IIIB strain) multiplication in acutely infected cells was based on the inhibition of virus-induced cytopathogenicity in MT-4 cells. Briefly, culture medium (50 μL) containing 1×104 cells was added to each well of flat-bottomed microtiter trays containing culture medium (50 μL) with or without various concentrations of the tested compounds and then a HIV-1 suspension (20 μL) containing 100 CCID50 (50% cell culture infective dose) was added. After 5 days of incubation at 37°C, the number of viable cells was determined by the MTT method. The cytotoxity of the compounds was evaluated in parallel with their antiviral activity, based on the viability of mock-infected cells as monitored by the MTT method.
HIV-1 RT inhibition assay
Inhibition of HIV-1 RT was developed using nucleotides linked to microtiter plate with colorimetric detection of incorporated biotin-dUTP into homopolymer template primers [
13]. The incorporated quantities of the biotin-dUTP into the enzyme represented the activity of HIV-1 RT. IC
50 values corresponded to the concentration of the substituted s-triazole derivatives required to inhibit biotin-dUTP incorporation into the HIV-1 RT by 50%.