New Polyhydroxylated Furostanol Saponins with Inhibitory Action against NO Production from Tupistra chinensis Rhizomes
Abstract
:Introduction
Results and Discussion
 : -60.7° (CH3OH; c 0.94). Positive coloration reactions were observed when 1 was subjected to Ehrlich, Molish and Liebermann-Buchard tests, which suggested that 1 had a furostanol saponin skeleton. Its molecular formula was established as C33H52O15 by its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 711.3208 [M+Na]+ (calcd. 711.3204 for C33H52O15Na). The molecular formula was further confirmed by the broad band and DEPT 13C-NMR spectra, which showed 33 signals comprising three methyl, eight methylene, sixteen methine and six quaternary carbons (Table 1). The downfield signal at δ 210.02 could be assigned as a carbonyl carbon and the presence of an olefinic bond could be deduced from a quaternary signal at δ 144.12 and a methylene signal at δ 112.73. The hemiketal carbon in the aglycone could be inferred by the signal at δ 110.79 [23]. A downfield signal at δ 80.78 could be assigned to the carbon bearing hydroxyl group located at furan ring of aglycone [24]. A methylene signal at δ 71.60 is a typical glycosidation carbon neighboring to an olefinic bond [25], ascribable to C-26 of the side chain. A methine carbon signal at δ 60.70 could be assigned to C-17 of aglycone. The presence of three methyl groups could be inferred by signals at δ 14.90, 10.87 and 14.00, respectively. The 1H-NMR data of 1 contained a methenyl proton at δ 4.68 (m), a doublet methyl group at δ 1.07 (d, J = 6.5 Hz) and two singlet methyl groups at δ 0.88 and 1.08 (each s), two typical protons bonded to ending olefinic carbon at δ 5.20 and 5.11 (each 1H, br s), attributable to a steroidal aglycone moiety [26]. Furthermore, the furostanol glycosidic nature of 1 was confirmed by the strong absorption bands at 3410 and 1050 cm-1 in the IR spectrum, and the signal due to semiketal carbon at δ110.79 in the 13C-NMR spectrum [23]. Upon acid hydrolysis of 1 with 2.0 mol/L HCl, only glucose was detected on thin layer chromatography and paper chromatography in the hydrolyzed product. Besides the spectral data due to aglycone, a group of downfield proton signals due to a hexose moiety were observed at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, d, J = 8.0 Hz), 3.48 (1H, m), 3.53 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.87 (1H, d, J = 12.5 Hz) and 3.76 (1H, dd, J = 12.5, 8.0 Hz) in the 1H-NMR spectrum, and the corresponding carbon signals were observed at δ 100.56, 74.01,75.34, 69.24, 75.41 and 60.33 in the HMQC spectrum. The signal due to the anomeric carbon at δ 100.56 showed a correlation with the signal due to H-26 at δ 4.41, and the signal due to the anomeric proton at δ 4.51 showed a correlation with the signal due to C-26 at δ 71.60 in the HMBC spectrum, which indicated that the sugar moiety was attached to C-26 of aglycone. Comparison of the 13C-NMR spectral data of 1 with those of 5β-furost-Δ25(27)-en-22-methoxyl-1β,2β,3β,4β,5β,7α,26-octaol-6-one 26-O-β-D-glucopyranoside [3] suggested that their chemical shifts were in good agreement, except for those due to C-20, C-22 and C-23, which resulted from the methylation of 22-hydroxyl group. Accordingly, 1 was identified as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,7α,22ξ,26-octaol-6-one 26-O-β-D-glucopyranoside. This structure was additionally confirmed by 2D NMR experiments, including 1H-1H COSY, TOCSY, NOESY, HMQC and HMBC.
: -60.7° (CH3OH; c 0.94). Positive coloration reactions were observed when 1 was subjected to Ehrlich, Molish and Liebermann-Buchard tests, which suggested that 1 had a furostanol saponin skeleton. Its molecular formula was established as C33H52O15 by its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 711.3208 [M+Na]+ (calcd. 711.3204 for C33H52O15Na). The molecular formula was further confirmed by the broad band and DEPT 13C-NMR spectra, which showed 33 signals comprising three methyl, eight methylene, sixteen methine and six quaternary carbons (Table 1). The downfield signal at δ 210.02 could be assigned as a carbonyl carbon and the presence of an olefinic bond could be deduced from a quaternary signal at δ 144.12 and a methylene signal at δ 112.73. The hemiketal carbon in the aglycone could be inferred by the signal at δ 110.79 [23]. A downfield signal at δ 80.78 could be assigned to the carbon bearing hydroxyl group located at furan ring of aglycone [24]. A methylene signal at δ 71.60 is a typical glycosidation carbon neighboring to an olefinic bond [25], ascribable to C-26 of the side chain. A methine carbon signal at δ 60.70 could be assigned to C-17 of aglycone. The presence of three methyl groups could be inferred by signals at δ 14.90, 10.87 and 14.00, respectively. The 1H-NMR data of 1 contained a methenyl proton at δ 4.68 (m), a doublet methyl group at δ 1.07 (d, J = 6.5 Hz) and two singlet methyl groups at δ 0.88 and 1.08 (each s), two typical protons bonded to ending olefinic carbon at δ 5.20 and 5.11 (each 1H, br s), attributable to a steroidal aglycone moiety [26]. Furthermore, the furostanol glycosidic nature of 1 was confirmed by the strong absorption bands at 3410 and 1050 cm-1 in the IR spectrum, and the signal due to semiketal carbon at δ110.79 in the 13C-NMR spectrum [23]. Upon acid hydrolysis of 1 with 2.0 mol/L HCl, only glucose was detected on thin layer chromatography and paper chromatography in the hydrolyzed product. Besides the spectral data due to aglycone, a group of downfield proton signals due to a hexose moiety were observed at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, d, J = 8.0 Hz), 3.48 (1H, m), 3.53 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.87 (1H, d, J = 12.5 Hz) and 3.76 (1H, dd, J = 12.5, 8.0 Hz) in the 1H-NMR spectrum, and the corresponding carbon signals were observed at δ 100.56, 74.01,75.34, 69.24, 75.41 and 60.33 in the HMQC spectrum. The signal due to the anomeric carbon at δ 100.56 showed a correlation with the signal due to H-26 at δ 4.41, and the signal due to the anomeric proton at δ 4.51 showed a correlation with the signal due to C-26 at δ 71.60 in the HMBC spectrum, which indicated that the sugar moiety was attached to C-26 of aglycone. Comparison of the 13C-NMR spectral data of 1 with those of 5β-furost-Δ25(27)-en-22-methoxyl-1β,2β,3β,4β,5β,7α,26-octaol-6-one 26-O-β-D-glucopyranoside [3] suggested that their chemical shifts were in good agreement, except for those due to C-20, C-22 and C-23, which resulted from the methylation of 22-hydroxyl group. Accordingly, 1 was identified as 5β-furost-Δ25(27)-en-1β,2β,3β,4β,5β,7α,22ξ,26-octaol-6-one 26-O-β-D-glucopyranoside. This structure was additionally confirmed by 2D NMR experiments, including 1H-1H COSY, TOCSY, NOESY, HMQC and HMBC. 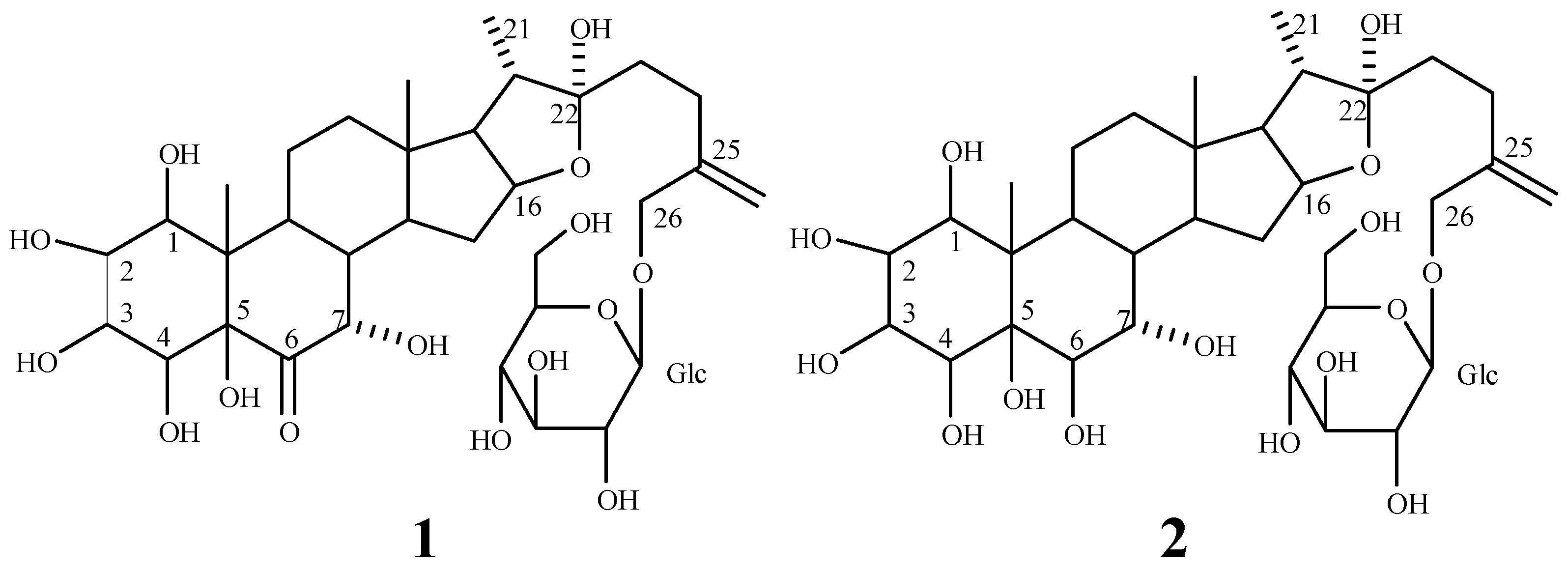
 : -27.0° (CH3OH; c 0.59). A skeleton of furostanol saponin was inferred by the positive coloration reactions of 1 with Ehrlich, Molish and Liebermann-Buchard reagents. This skeleton was confirmed by the strong absorption bands at 3406 cm-1 and 1056 cm-1 in the IR spectrum and the signal due to a semiketal carbon (C-22) at δ 111.34 in the 13C-NMR spectrum [23]. Its molecular formula was deduced as C33H54O15 from its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 713.3367 [M+Na]+ (calcd. 713.3360 for C33H54O15Na). This formula was in good agreement with the broad band and DEPT 13C-NMR spectra, which showed 33 signals containing three methyl, eight methylene, seventeen methine and five quaternary carbons (Table 1). A downfield signal due to methine carbon at δ 81.52 showed correlation with a downfield signal at δH 4.66, attributable to H-16, which showed a correlation with semiketal signal at δ 111.34 in the HMBC spectrum. The presence of a terminal olefinic bond was inferred by the signal due to a quaternary carbon at δ 144.67, as well as the signal due to a methylene carbon at δ 113.26, which showed correlations with two olefinic proton signals at δ 5.20 and 5.18 (each 1H, br s) in the HMQC spectrum. A methylene carbon signal at δ 71.96 showed correlation with signals at δH 4.41 (1H, d, J = 13.0 Hz) and 4.25 (1H, d, J =13.0 Hz), ascribable to H-26. H-26 showed connectivity with an anomeric carbon signal at δ 101.10 and the olefinic carbon signal at δ 144.67 in the HMBC spectrum. A β-D-glucopyranosyl moiety was inferred by the facts that a group of proton signals at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, dd, J = 10.5, 8.0 Hz), 3.47 (1H, m), 3.54 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.97 (1H, d, J = 12.5 Hz) and 3.77 (1H, dd, J = 12.5, 8.0 Hz) showed correlations with a group of carbon signals at δ 101.10, 73.78, 75.52, 69.29, 75.94 and 60.88, respectively, in the HMQC spectrum. This group of proton signals showed correlations with each other in the TOCSY spectrum.
: -27.0° (CH3OH; c 0.59). A skeleton of furostanol saponin was inferred by the positive coloration reactions of 1 with Ehrlich, Molish and Liebermann-Buchard reagents. This skeleton was confirmed by the strong absorption bands at 3406 cm-1 and 1056 cm-1 in the IR spectrum and the signal due to a semiketal carbon (C-22) at δ 111.34 in the 13C-NMR spectrum [23]. Its molecular formula was deduced as C33H54O15 from its HR-FAB-MS spectrum in positive ion mode, which showed a quasi-molecular ion peak at m/z 713.3367 [M+Na]+ (calcd. 713.3360 for C33H54O15Na). This formula was in good agreement with the broad band and DEPT 13C-NMR spectra, which showed 33 signals containing three methyl, eight methylene, seventeen methine and five quaternary carbons (Table 1). A downfield signal due to methine carbon at δ 81.52 showed correlation with a downfield signal at δH 4.66, attributable to H-16, which showed a correlation with semiketal signal at δ 111.34 in the HMBC spectrum. The presence of a terminal olefinic bond was inferred by the signal due to a quaternary carbon at δ 144.67, as well as the signal due to a methylene carbon at δ 113.26, which showed correlations with two olefinic proton signals at δ 5.20 and 5.18 (each 1H, br s) in the HMQC spectrum. A methylene carbon signal at δ 71.96 showed correlation with signals at δH 4.41 (1H, d, J = 13.0 Hz) and 4.25 (1H, d, J =13.0 Hz), ascribable to H-26. H-26 showed connectivity with an anomeric carbon signal at δ 101.10 and the olefinic carbon signal at δ 144.67 in the HMBC spectrum. A β-D-glucopyranosyl moiety was inferred by the facts that a group of proton signals at δ 4.51 (1H, d, J = 8.0 Hz), 3.43 (1H, dd, J = 10.5, 8.0 Hz), 3.47 (1H, m), 3.54 (1H, t, J = 9.0 Hz), 3.35 (1H, dd, J = 9.0, 8.0 Hz), 3.97 (1H, d, J = 12.5 Hz) and 3.77 (1H, dd, J = 12.5, 8.0 Hz) showed correlations with a group of carbon signals at δ 101.10, 73.78, 75.52, 69.29, 75.94 and 60.88, respectively, in the HMQC spectrum. This group of proton signals showed correlations with each other in the TOCSY spectrum. | Position | 1 | 2 | ||||
| 13C | DEPT | 1H (J, Hz) | 13C | DEPT | 1H (J, Hz) | |
| 1 | 74.08 | CH | 3.95, d, (2.0) | 77.01 | CH | 3.88, br s |
| 2 | 65.68 | CH | 3.97, d, (2.0) | 65.24 | CH | 3.90, br s |
| 3 | 72.97 | CH | 4.22, d, (3.5) | 72.15 | CH | 4.18, d, (3.0) |
| 4 | 68.89 | CH | 5.03, d, (3.5) | 68.48 | CH | 4.43, d, (3.0) |
| 5 | 84.86 | C | 77.93 | C | ||
| 6 | 210.02 | C | 73.20 | CH | 4.80, br s | |
| 7 | 72.66 | CH | 3.53, d, (9.0) | 69.78 | CH | 4.12, d, (2.5) |
| 8 | 36.69 | CH | 2.18, br t, (9.0) | 33.03 | CH | 2.16, m |
| 9 | 40.19 | CH | 2.20, br t, (9.0) | 36.81 | CH | 2.31, m |
| 10 | 49.00 | C | 45.41 | C | ||
| 11 | 20.50 | CH2 | 1.38, m | 20.35 | CH2 | 1.36, m |
| 12 | 38.93 | CH2 | 1.60, m; 1.22, m | 38.55 | CH2 | 1.60, td, (13.0, 5.0); 1.21, td, (13.0, 5.0) |
| 13 | 39.77 | C | 39.62 | C | ||
| 14 | 47.76 | CH | 1.96, m | 48.76 | CH | 1.96, m |
| 15 | 29.69 | CH2 | 2.14, m; 1.48, m | 30.61 | CH2 | 2.15, m; 1.45, m |
| 16 | 80.78 | CH | 4.68, m | 81.52 | CH | 4.66, m |
| 17 | 60.70 | CH | 1.66, m | 61.37 | CH | 1.66, m |
| 18 | 14.90 | CH3 | 0.88, s | 15.42 | CH3 | 0.89, s |
| 19 | 10.87 | CH3 | 1.08, s | 14.15 | CH3 | 1.39, s |
| 20 | 40.19 | CH | 1.82, m | 40.34 | CH | 1.83, m |
| 21 | 14.00 | CH3 | 1.07, d, (6.5) | 14.57 | CH3 | 1.08, d, (6.5) |
| 22 | 110.79 | C | 111.34 | C | ||
| 23 | 35.09 | CH2 | 1.87, m; 1.63, m | 35.64 | CH2 | 1.85, m; 1.62, m |
| 24 | 26.29 | CH2 | 2.28, m; 1.98, m | 26.82 | CH2 | 2.30, m; 1.95, m |
| 25 | 144.12 | C | 144.67 | C | ||
| 26 | 71.60 | CH2 | 4.41, d, (12.5, H-ax); 4.25, d, (12.5, H-eq) | 71.96 | CH2 | 4.41, d, (13.0, H-ax); 4.25, d, (13.0, H-eq) |
| 27 | 112.73 | CH2 | 5.20, br s, H-a; 5.11, br s, H-b | 113.26 | CH2 | 5.20, br s, H-a; 5.11, br s, H-b |
| Glc | ||||||
| 1 | 100.56 | CH | 4.51, d, (8.0) | 101.10 | CH | 4.51, d, (8.0) |
| 2 | 74.01 | CH | 3.43, br d, (8.0) | 73.78 | CH | 3.43, dd, (10.5, 8.0) |
| 3 | 75.34 | CH | 3.48, m | 75.52 | CH | 3.47, m |
| 4 | 69.24 | CH | 3.53, t, (9.0) | 69.29 | CH | 3.54, t, (9.0) |
| 5 | 75.41 | CH | 3.35, ddd, (9.0, 5.5, 2.5) | 75.94 | CH | 3.35, ddd, (9.0, 5.5, 2.2) |
| 6 | 60.33 | CH2 | 3.87, dd, (12.5, 2.5); 3.76, dd, (12.5, 5.5) | 60.88 | CH2 | 3.88,dd, (12.5, 2.2); 3.77, dd, (12.5, 5.5) |
Biological activity
| Groups | Dosages (μg/mL) | NO content μmol/L | |
|---|---|---|---|
| Blank a) | — | 53.62 ± 7.83 | |
| Model b) | — | 147.35 ± 15.20 | |
| Aspirin c) | 40 | 106.86 ± 4.15 ** g) | |
| n-Butanol soluble fraction d) | 20 | 110.29 ± 58.08 ** | |
| Water soluble fraction e) | 20 | 94.61 ± 26.38 ** | |
| 60% methanolic eluate f) | 20 | 85.78 ± 41.87 ** | |
| Compound 1 | 40 | 87.34 ± 7.33 ** | |
| Compound 2 | 40 | 108.34 ± 11.49 ** | |
Conclusions
Experimental
General
Plant Material
Extraction and Isolation
 : -60.7° (CH3OH; c 0.94); IR (KBr) cm-1: 3410, 2923, 1708, 1655, 1443, 1050; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 711.3208 [M+Na]+, calcd for C33H52O15Na, 711.3204.
: -60.7° (CH3OH; c 0.94); IR (KBr) cm-1: 3410, 2923, 1708, 1655, 1443, 1050; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 711.3208 [M+Na]+, calcd for C33H52O15Na, 711.3204. : -27.0° (CH3OH; c 0.59); IR (KBr) cm-1: 3406, 2923, 1655, 1445, 1056; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 713.3367 [M+Na]+, calcd for C33H54O15Na, 713.3360.
: -27.0° (CH3OH; c 0.59); IR (KBr) cm-1: 3406, 2923, 1655, 1445, 1056; 1H-NMR and 13C-NMR: see Table 1; HR-FAB-MS (positive ion mode): m/z 713.3367 [M+Na]+, calcd for C33H54O15Na, 713.3360.Acid Hydrolysis
Bioassay
Acknowledgements
References and Notes
- Zhan, Y.H. China Shennongjia Resources of Medicinal Plants; Hubei Scientific and Technological Press: Wuhan, 1994; p. 418. [Google Scholar]
- Jiangsu New Medical College. Dictionary of Traditional Chinese Medicines; Shanghai Scientific and Technological Press: Shanghai, 1979; p. 907. [Google Scholar]
- Wendehenne, D.; Pugin, A.; Klessig, D.; Durner, J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Shen, S.C.; Lee, W.R.; Lin, H.Y.; Huang, H.C.; Ko, C.H.; Yang, L.L. In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E2 productions. Eur. J. Pharmacol. 2002, 446, 187–194. [Google Scholar] [CrossRef]
- Chang, Y.C.; Li, P.C.; Chen, B.C.; Chang, M.S.; Wang, J.L.; Chiu, W.T. Lipoteichoic acid-induced nitric oxide synthase expression in RAW 264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-kappaB pathways. Cell Signal 2006, 18, 1235–1243. [Google Scholar] [CrossRef]
- Jiang, W.; Pisetsky, D.S. The role of IFN-alpha and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic–polycytidylic acid or lipopolysaccharide. J. Immunol. 2006, 177, 3337–3343. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zou, K.; Zhang, Y.M.; Liu, C.; Wu, J.; Zhou, Y.; Dan, F.J.; Zhang, Y.X. An 18-norspirostanol saponin with inhibitory action against COX-2 production from the underground part of Trillium tschonoskii. Chem. Pharm. Bull. 2007, 55, 679–681. [Google Scholar] [CrossRef]
- Zou, K.; Wu, J.; Du, M.; Liu, C. Diastereoisomeric saponins from Tupistra chinensis rhizomes. Chin. Chem. Lett. 2007, 18, 65–68. [Google Scholar] [CrossRef]
- Tang, Z.C.; Zou, K.; Wang, J.Z.; Yin, C.Z.; Lu, H.H. Antialcoholism of extracts from Tupistra chinensis rhizomes. Lishizhen Med. Materia Med. Res. 2006, 17, 2163–2165. [Google Scholar]
- Wang, Z.J.; Zou, K.; Xu, H.W. Anti-inflammatory effects of Tupistra chinensis rhizomes. Lishizhen Med. Materia Med. Res. 2006, 17, 1970–1971. [Google Scholar]
- Yang, C.Y.; Zou, K.; Pan, J.R. Volatile constituents from Tupistra chinensis rhizomes. J. Chin. Three Gorges Univ. (Nat. Sci. Ed.) 2006, 28, 360–362. [Google Scholar]
- Zou, K.; Tu, G.Z.; Yang, C.Y.; Wang, J.Z. A furostanol saponin from the cytotoxic extract of Tupistra chinensis rhizomes. Chin. Chem. Lett. 2006, 17, 1335–1338. [Google Scholar]
- Zou, K.; Wang, J.Z.; Wu, J.; Liu, C.; Xiao, Z.H. A pair of diastereoisomeric saponins from the cytotoxic extract of Tupistra chinensis rhizomes. Chem. Pharm. Bull. 2006, 54, 1782–1785. [Google Scholar]
- Liu, Z.X.; Zou, K.; Yang, X.H.; Zhou, Y. Anti-inflammatory and analgesic effects of ethanol extracts from roots of Panax japonicus. Lishizhen Med. Materia Med. Res. 2004, 15, 465–466. [Google Scholar]
- Liu, Z.X.; Zou, K. A survey on the study of Panax plants in Hubei Province. Lishizhen Med. Materia Med. Res. 2003, 14, 571–573. [Google Scholar]
- Huang, L.; Liao, Q.B.; Zou, K.; Yuan, H.; Hu, C.L.; Hu, Y.L. Content determination of steroidal sapogenins in Tupistra chinensis rhizomes. J. Chin. Three Gorges Univ. (Nat. Sci. Ed.) 2003, 25, 562–564. [Google Scholar]
- Gao, Z.; Zou, K.; Liao, Q.B. Scavenging effects of Dipsacus asper Wall on DPPH radical group. J. Chin. Three Gorges Univ. (Nat. Sci. Ed.) 2002, 24, 366–368. [Google Scholar]
- Pan, W.B.; Wei, L.M.; Wei, L.L.; Wu, C.Y. Chemical Constituents of Tupistra chinensis Rhizomes. Chem. Pharm. Bull. 2006, 54, 954–958. [Google Scholar] [CrossRef]
- Wu, G.X.; Wei, X.Y.; Chen, W.X. Spirostane steroidal saponins from the underground parts of Tupistra chinensis. Chin. Chem. Lett. 2005, 16, 911–914. [Google Scholar]
- Pan, W.B.; Chang, F.R.; Wu, Y.C. Tupichigenin A, a new steroidal sapogenin from Tupistra chinensis. J. Nat. Prod. 2000, 63, 861–863. [Google Scholar] [CrossRef]
- Pan, W.B.; Chang, F.R.; Wei, L.M.; Wu, Y.C. New flavans, spirostanol sapogenins, and a pregnane genin from Tupistra chinensis. J. Nat. Prod. 2003, 66, 161–168. [Google Scholar] [CrossRef]
- Pan, W.B.; Chang, F.R.; Wu, Y.C. Spirostanol sapogenins from the underground parts of Tupistra chinensis. Chem. Pharm. Bull. 2000, 48, 1350–1353. [Google Scholar] [CrossRef] [Green Version]
- Dini, I.; Tenore, G.C.; Trimarco, E.; Dini, A. Furostanol saponins in Allium caepa L. Var. tropeana seeds. Food Chem. 2005, 93, 205–214. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Gupta, R.K.; Thakur, R.S. Carbon-13 NMR spectroscopy of steroidal sapogenins and steroidal saponins. Phytochemistry 1985, 24, 2479–2496. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Pathak, A.K. NMR spectroscopy of steroidal sapogenins and steroidal saponins: an update. Magn. Reson. Chem. 1995, 33, 923–953. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Takashi, Y.; Sashida, Y. Steroidal saponins from the leaves of Cordyline stricta. Phytochemistry 1998, 47, 79–85. [Google Scholar] [CrossRef]
- Shen, P. Biological constituents from Dioscorea deltoidea Wall var. orbiculata and Tupistra wattii Hook.f. Doctoral Thesis, Shengyang Pharmaceutical University, 2002; pp. 39–43. [Google Scholar]
- Chen, Y.; Liu, J.; Xie, Z. Progress in studies on Tupistra species. Lishizhen J. Med. Materia Med. Res. 2004, 15, 860–861. [Google Scholar]
- Yang, C.Y.; Yang, X.H.; Zou, K.; Liu, Y.; Yue, C.Y. Effects of Tupistra chinensis rhizomes on phlegm, inflammation and bacteria. Chin. J. Folk Med. Materia Med. Res. 2005, 73, 103–106. [Google Scholar]
- Qiu, J.; Dong, W.G. Inhibitory mechanism of extracts from Tupistra chinensis rhizomes on colonitis. J. Shangdong Med. Materia Med. Res. 2005, 45, 4–6. [Google Scholar]
- Qiu, J.; Dong, W.G.; Yu, J.P. Effects of extracts from Tupistra chinensis rhizomes on activity of platelet in rats suffering from colocitis. Chin. J. Comb. Chin. Med. West. Med. Digest. 2005, 13, 363–365. [Google Scholar]
- Li, X.L.; Zhang, Y.Q.; Hong, P.P. Anti-inflammatory and analgesic effects of Tupistra chinensis rhizomes. J. Hubei Chin. Med. Inst. 2005, 7, 28–29. [Google Scholar]
- Chu, S.S. Curative effects of Tupistra chinensis rhizomes on pharyngitis and faucitis. Chin. J. Folk Med. Materia Med. Res. 1999, 38, 140–141. [Google Scholar]
- Zou, K.; Tong, W.Y.; Liang, H.; Cui, J.R.; Tu, G.Z.; Zhao, Y.Y.; Zhang, R.Y. Diastereoisomeric saponins from Albizia julibrissin. Carbohydr. Res. 2005, 340, 1329–1334. [Google Scholar] [CrossRef]
- Sample Availability: available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Xu, L.-L.; Zou, K.; Wang, J.-Z.; Wu, J.; Zhou, Y.; Dan, F.-J.; Yang, J. New Polyhydroxylated Furostanol Saponins with Inhibitory Action against NO Production from Tupistra chinensis Rhizomes. Molecules 2007, 12, 2029-2037. https://doi.org/10.3390/12082029
Xu L-L, Zou K, Wang J-Z, Wu J, Zhou Y, Dan F-J, Yang J. New Polyhydroxylated Furostanol Saponins with Inhibitory Action against NO Production from Tupistra chinensis Rhizomes. Molecules. 2007; 12(8):2029-2037. https://doi.org/10.3390/12082029
Chicago/Turabian StyleXu, Lan-Lan, Kun Zou, Jun-Zhi Wang, Jun Wu, Yuan Zhou, Fei-Jun Dan, and Jing Yang. 2007. "New Polyhydroxylated Furostanol Saponins with Inhibitory Action against NO Production from Tupistra chinensis Rhizomes" Molecules 12, no. 8: 2029-2037. https://doi.org/10.3390/12082029





