Unusual Reactivity Patterns of 1,3,6,8-Tetraazatricyclo-[4.4.1.13,8]-dodecane (TATD) Towards Some Reducing Agents: Synthesis of TMEDA
Abstract
:Introduction

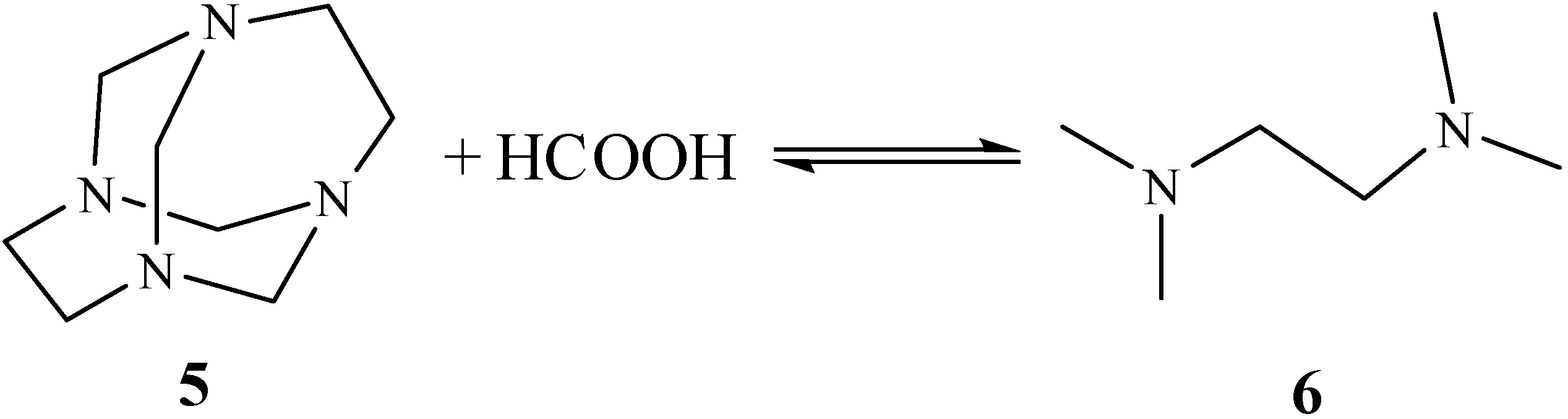
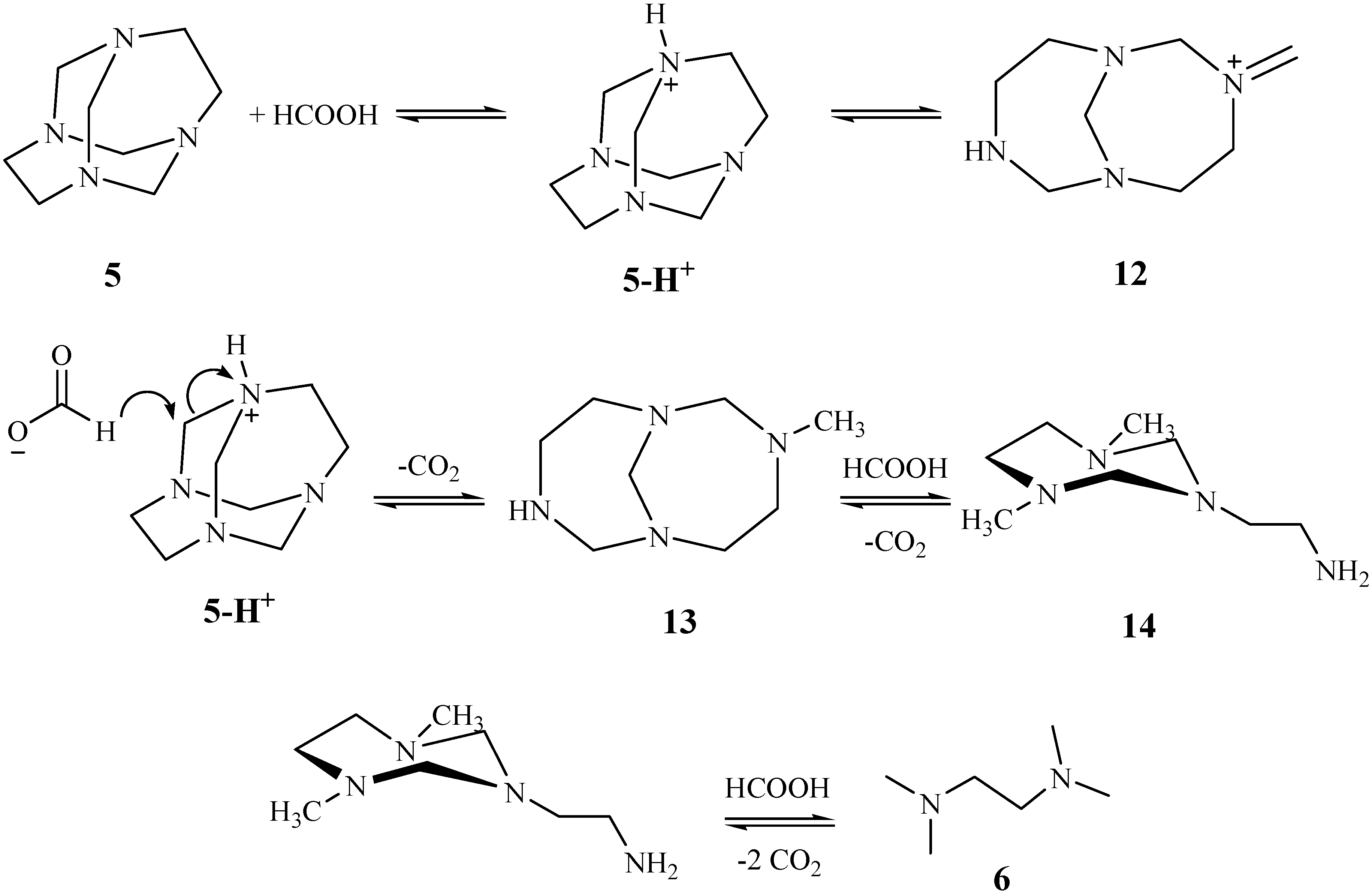
Results and Discussion



Conclusions
Experimental
General
Reduction of TATD with formic acid
Acknowledgments
References and Notes
- Boyd, E.; Coumbarides, G. S.; Eames, J.; Jones, R. V. H.; Stenson, R. A.; Suggate, M. J. Synthesis and derivatisation of N,N´-trisubstituted 1,2-diamines derived from (1R,2R)-1,2-diaminocyclohexane. Tetrahedron Lett. 2005, 46, 3479–3484. [Google Scholar]
- Patai, S. (Ed.) The Chemistry of Functional Groups, Supplement F, 2; John Wiley Sons: New York, 1982; pp. 895–897.
- Denat, F.; Tripier, R.; Boschetti, F.; Espinosa, E.; Guilard, R. Reaction of polyamines with diethyloxalate: A convenient route for the synthesis of tetraazacycloalkanes. Arkivoc 2006, 4, 212–233. [Google Scholar]
- Salerno, A.; Figueroa, M. A.; Perillo, I. A. A convenient ‘‘One-Pot’’ reaction for selective monoalkylation of N,N´-disubstituted ethylenediamines. Synth. Commun. 2003, 33, 3193–3204. [Google Scholar] [CrossRef]
- Alexakis, A.; Andrey, O. Diamine-catalyzed asymmetric Michael additions of aldehydes and ketones to nitrostyrene. Org. Lett. 2002, 4, 3611–3614. [Google Scholar] [CrossRef]
- March, J. Advanced Organic Chemistry, 3th ed.; Wiley Interscience: New York, 1985; p. 809, and references therein. [Google Scholar]
- Lennartson, A.; Hedström, A.; Håkansson, M. Diisopropyl(N,N,N´,N´-tetramethylethyle-nediamine)zinc(II), the first crystal structure of a diisopropylzinc complex. Acta Cryst. 2007, E63, 123–125. [Google Scholar] Pintauer, T.; Matyjaszewski, K. Structural aspects of copper catalyzed atom transfer radical polymerization. Coord. Chem. Rev. 2005, 249, 1155–1184. [Google Scholar] [CrossRef] Hitchcock, P. B.; Lee, T. H.; Leigh, G. J. N,N,N´,N´-tetramethylethane-1,2-diamine complexes of vanadium chlorides. Inorg. Chim. Act. 2003, 349, 159–164. [Google Scholar] [CrossRef] Daniele, S.; Hubert-Pfalzgraf, L. G.; Perrin, M. Molecular structures of volatile Ce(IV) tetrafluoroisopropoxide complexes with TMEDA and diglyme. CVD experiments. Polyhedron 2002, 21, 1985–1990. [Google Scholar] [CrossRef] Handley, D. A.; Hitchcock, P. B.; Lee, T.-H.; Leigh, G. J. Copper(II) adducts with N,N,N´,N´-tetramethylethane-1,2-diamine and attempts to prepare trinuclear derivatives. Inorg. Chim. Act. 2001, 316, 59–64. [Google Scholar] [CrossRef] Handley, D. A.; Hitchcock, P. B.; Leigh, G. J. Triangulo-pentahalotrimetal complexes of nickel(II) and cobalt(II) with N,N,N´,N´,N-tetramethylethane-1,2-diamine and related compounds. Inorg. Chim. Act. 2001, 314, 1–13. [Google Scholar] [CrossRef] Handley, D. A.; Hitchcock, P. B.; Lee, T. H.; Leigh, G. J. Complexes of metal(II) halides of the first transition series with N,N,N´,N´-tetramethylmethane-diamine,-ethane-1,2-diamine and -propane-1,3-diamine. Inorg. Chim. Act. 2001, 314, 14–21. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shibasaki, Y.; Ando, S.; Ueda, M. Synthesis of novel poly[(1,3-adaman-tyl)bis(2-naphthol)] with low dielectric constant. Polymer 2006, 47, 3043–3048. [Google Scholar] Ito, S.; Koizumi, K.; Fukuda, K.; Kameta, N.; Ikeda, T.; Oba, T.; Hiratani, K. Novel synthesis of macrocycles with 1,10-binaphthalene-2,20-diol using intramolecular oxidative coupling. Tetrahedron Lett. 2006, 47, 8563–8566. [Google Scholar] [CrossRef]
- Nielson, A. J.; Glenny, M. W.; Rickard, C. E. F. 2-tert-butyl and 2-phenylphenylimido complexes of titanium(IV) and their olefin polymerisation activity. J. Chem. Soc., Dalton Trans. 2001, 232–239. [Google Scholar] Zhang, X.; Xia, J.; Matyjaszewski, K. Controlled/“Living” radical polymerization of 2-(dimethylamino)ethyl methacrylate. Macromolecules 1998, 31, 5167–5169. [Google Scholar] [CrossRef] Xia, J.; Matyjaszewski, K. Controlled/“Living” radical polymerization. atom transfer radical polymerization using multidentate amine ligands. Macromolecules 1997, 30, 7697–7700. [Google Scholar] [CrossRef]
- Yasuda, H.; Choi, J.-C.; Lee, S.-C.; Sakakura, T. Reactivity of diaryloxy palladium complex with TMEDA (N,N,N´,N´-tetramethylethylenediamine) ligand toward carbon monoxide and carbon dioxide. Organometallics 2002, 21, 1216–1220. [Google Scholar] [CrossRef]
- Jones, G. D.; Martin, J. L.; McFarland, C.; Allen, O. R.; Hall, R. E.; Haley, A. D.; Brandon, R. J.; Konovalova, T.; Desrochers, P. J.; Pulay, P.; Vicic, D. A. Ligand redox effects in the synthesis, electronic structure, and reactivity of an alkyl-alkyl cross-coupling catalyst. J. Am. Chem. Soc. 2006, 128, 13175–13183. [Google Scholar]
- Halasa, A. F.; Prentis, J.; Hsu, B.; Jasiunas, C. High vinyl high styrene solution SBR. Polymer 2005, 46, 4166–4174. [Google Scholar] [CrossRef]
- Montagne, C.; Prévost, N.; Shiers, J. J.; Prié, G.; Rahman, S.; Hayes, J. F.; Shipma, M. Generation and electrophilic substitution reactions of 3-lithio-2-methyleneaziridines. Tetrahedron 2006, 62, 8447–8457. [Google Scholar] [CrossRef] Martineau, D.; Gros, P.; Fort, Y. Selective lithiation of 4-(1H-1-pyrrolyl)pyridine. Access to new electron-releasing ligands. J. Org. Chem. 2004, 69, 7914–7918. [Google Scholar] [CrossRef] Rutherford, J. L.; Hoffmann, D.; Collum, D. B. Consequences of correlated solvation on the structures and reactivities of RLi-diamine complexes: 1,2-Addition and α-lithiation reactions of imines by TMEDA-solvated n-butyllithium and phenyllithium. J. Am. Chem. Soc. 2002, 124, 264–271. [Google Scholar] [CrossRef] Chadwick, S. T.; Rennels, R. A.; Rutherford, J. L.; Collum, D. B. Are n-BuLi/TMEDA-mediated arene ortholithiations directed? Substituent-dependent rates, substituent-independent mechanisms. J. Am. Chem. Soc. 2000, 122, 8640–8647. [Google Scholar] [CrossRef] Workentin, M. S.; Johnston, L. J.; Wayner, D. D. M.; Parker, V. D. Reactivity of aromatic radical cations. rate constants for reactions of 9-phenyl- and 9,lO-diphenylanthracene radical cations with acyclic amines1. J. Am. Chem. Soc. 1994, 116, 8279–8287. [Google Scholar] [CrossRef] Ager, D. J.; East, M. B. A comparison of the reactions of [(phenylthio) (trimethylsily1)methylllithium with α,β-unsaturated ketones and those of other acyl anion equivalents containing sulfur1. J. Org. Chem. 1986, 51, 3983–3992. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Xu, S.; Song, H.; Wang, B. A general synthetic route to 6,6-substituted-6H-dibenzo[b,d]pyrans from dibenzofuran. J. Org. Chem. 2006, 71, 8291–8293. [Google Scholar]
- Brown, H. C.; Dhokte, U. P. Hydroboration. 91. Improved procedure for the synthesis of optically pure bis-adducts, N,N,N,'N-Tetramethylethylenediamine-2-organylapoisopinocam-pheylboranes, from the corresponding 2-organylapopinenes of lower optical purity. Conversion of these adducts into 2-organylapoisopinocampheylboranes, useful asymmetric hydroborating reagents. J. Org. Chem. 1994, 59, 2365–2369. [Google Scholar]
- Kennedy, A.; Nelson, A.; Perry, A. Methods for the synthesis of polyhydroxylated piperidines by diastereoselective dihydroxylation: Exploitation in the two-directional synthesis of aza-C-linked disaccharide derivatives. Beilstein J. Org. Chem. 2005, 1, 1–10. [Google Scholar] Donohoe, T. J.; Blades, K.; Moore, P. R.; Waring, M. J.; Winter, J. J. G.; Helliwell, M.; Newcombe, N. J.; Stemp, G. Directed dihydroxylation of cyclic allylic alcohols and trichloroacetamides Using OsO4/TMEDA. J. Org. Chem. 2002, 67, 7946–7956. [Google Scholar] [CrossRef] Donohoe, T. J.; Blades, K.; Helliwell, M.; Waring, M. J.; Newcombe, N. J. The synthesis of (+)-pericosine B. Tetrahedron Lett. 1998, 39, 8755–8758. [Google Scholar] [CrossRef]
- Beilsteins Handbuch der Organischen Chemie; Prager, B.; Jacobson, P. (Eds.) Springer: Berlin, 1918; Band IV; p. 250. Marsella, J. A. Eur. Patent 169547, 1986. U.S. Patent 3,634,503, 1984, [Chem. Abs. 1986, 105, 174780f]. Aresta, M.; De Fazio, M.; Bruno, P. A Study of the system [Rh(C2H4)2Cl]2-P[N(CH3)2]3 and evidence of N,N,N',N'-tetramethylethylenediamine formation via intramolecular N(CH3)2 transfer to η2-C2H4. Reaction with CO2 of the rhodium complexes. Inorg. Chem. 1982, 21, 441–444. [Google Scholar] [CrossRef]
- Aldridge, S.; Downs, A. J. Hydrides of the main-group metals: New variations on an old theme. Chem. Rev. 2001, 101, 3305–3365. [Google Scholar] [CrossRef] Blais, P.; Brask, J. K.; Chivers, T.; Schatte, G. Tetraaza analogues of lithium and sodium alums: Synthesis and X-ray structures of the single strand polymer [Li(THF)2{Al[SO2(NtBu)2]2}]∞ and the contact ion pairs [Na(15-crown-5)][Al{SO2(NtBu)2}2] and {[Na(15-crown-5)][O2S(í-NBn)2Al(í-NBnSO2NBn)]}2. Inorg. Chem. 2001, 40, 384–388. [Google Scholar] [CrossRef] Pauls, J.; Neumiiller, B. Lithium amidohydridoaluminates. Inorg. Chem. 2001, 40, 121–124. [Google Scholar] [CrossRef] Emig, N.; Nguyen, H.; Krautscheid, H.; Réau, R.; Cazaux, J.-B.; Bertrand, G. Neutral and cationic tetracoordinated aluminum complexes featuring tridentate nitrogen donors: Synthesis, structure, and catalytic activity for the ring-opening polymerization of propylene oxide and (D,L)-Lactide. Organometallics 1998, 17, 3599–3608. [Google Scholar] [CrossRef] Gardiner, M. G.; Raston, C. L:. Advances in the chemistry of the Lewis base adducts of alane and gallane. Coord. Chem. Rev. 1997, 166, 1–34. [Google Scholar] [CrossRef] Atwood, J. L.; Bennett, F. R.; Elms, F. M.; Jones, C.; Raston, C. L.; Robinson, K. D. Tertiary amine stabilized dialane. J. Am. Chem. Soc. 1991, 113, 8183–8185. [Google Scholar] Dilts, J. A.; Ashby, E. C. The composition of complex metal hydrides in polar solvents. I. Tertiary amines. Inorg. Chem. 1970, 9, 855–862. [Google Scholar] [CrossRef] Ehrlich, R.; Rice, G. The chemistry of alane. XI11. The lithium tetrahydroalanate-triethylamine complex. Inorg. Chem. 1966, 5, 1284–1286. [Google Scholar] [CrossRef]
- Chouzier, S.; Afanasiev, P.; Vrinat, M.; Cseri, T.; Roy-Auberger, M. One-step synthesis of dispersed bimetallic carbides and nitrides from transition metals hexamethylenetetramine complexes. J. Solid. State Chem. 2006, 179, 3314–3323. [Google Scholar] [CrossRef] Konar, S.; Mukherjee, P. S.; Drew, M.G. B.; Ribas, J.; Chaudhuri, N. R. Syntheses of two new 1D and 3D networks of Cu(II) and Co(II) using malonate and urotropine as bridging ligands: Crystal structures and magnetic studies. Inorg. Chem. 2003, 42, 2545–2552. [Google Scholar] [CrossRef] Konar, S.; Zangrando, E.; Chaudhuri, N. R. Combination of covalent and hydrogen bonding in the formation of 3D Co(II)/fumarate networks. Inorg. Chim. Act. 2003, 355, 264–271. [Google Scholar] [CrossRef] Graham, P. M.; Pike, R. D.; Sabat, M.; Bailey, R. D.; Pennington, W. T. Coordination Polymers of copper(I) halides. Inorg. Chem. 2000, 39, 5121–5132. [Google Scholar] [CrossRef] Stocker, F. B.; Staeva, T. P.; Rienstra, C. M.; Britton, D. Crystal structures of a series of complexes produced by reaction of copper(I) cyanide with diamines. Inorg. Chem. 1999, 38, 984–991. [Google Scholar] [CrossRef]
- Nygren, C. L.; Wilson, C. C.; Turner, J. F. C. Electron and Nuclear Positions in the Short Hydrogen Bond in Urotropine-N-oxide·Formic Acid. J. Phys. Chem. A. 2005, 109, 1911–1919. [Google Scholar] [CrossRef] Mak, T. C. W.; Ladd, M. F. C.; Povey, D. C. Molecular and crystal structure of hexamethylenetetramine oxide. J. Chem. Soc., Perkin Trans. 2 1979, 593–595. [Google Scholar]
- Henry, R. A.; Hollins, R. A.; Lowe-Ma, C.; Moore, D. W.; Nissan, R. A. Anomalous reaction of pentafluorophenacyl bromide with hexamethylenetetramine. Structure of the product1. J. Org. Chem. 1990, 55, 1796–1801. [Google Scholar] [CrossRef] Ribár, B.; Mészarós, C.; Vladimirov, S.; Živanov-Stakić, D.; Golič, L. Structure of N-methylurotropinium iodide. Acta Crystallogr. 1991, C47, 1987–1989. [Google Scholar] Bahner, C. T.; Pickens, M. D.; Pickens, D.; Easley, W. K:. Some quaternary salts of hexamethylenetetramine. J. Am. Chem. Soc. 1950, 72, 2266–2267. [Google Scholar] [CrossRef]
- Riley, M. D.; Miller, N. E. Hexamethylenetetramine-borane adducts. Inorg. Chem. 1974, 13, 707–710. [Google Scholar]
- Kondo, H.; Kodama, G. Reactions of hexamethylenetetramine with diborane(6), triborane(7), tetraborane(l0), and pentaborane(11). Inorg. Chem. 1979, 18, 1460–1464. [Google Scholar]
- Rivera, A.; González-Salas, D.; Ríos-Motta, J.; Hernández-Barragán, A.; Joseph-Nathan, P. Preferred hydrogen bonding site of 1,3,6,8-tetraazatricyclo[4.3.1.13,8]undecane (TATU) to hydroquinone. J. Mol. Struct. 2007, 837, 142–146. [Google Scholar] [CrossRef]
- Clarke, H. T.; Gillespie, H. B.; Weisshaus, S. Z. The Action of formaldehyde on amines and amino Acids. J. Am. Chem. Soc. 1933, 55, 4571–4587. [Google Scholar] [CrossRef]
- Gibson, H. W. The chemistry of formic acid and its simple derivatives. Chem Rev. 1969, 69, 673–692. [Google Scholar] [CrossRef]
- Lukasiewicz, A. A study of the mechanism of certain chemical reactions-I: The mechanism of the Leuckart-Wallach reaction and of the reduction of schiff bases by formic acid. Tetrahedron 1963, 19, 1789–1799. [Google Scholar] [CrossRef] Pollard, C. B.; Young, Jr. D. C. The mechanism of the Leuckart reaction. J. Org. Chem. 1951, 16, 661–672. [Google Scholar] [CrossRef] Staple, E.; Wagner, E. C. A study of the Wallach reaction for alkylation of amines by action of aldehydes or ketones and formic acid1. J. Org. Chem. 1949, 71, 559–578. [Google Scholar] [CrossRef] deBenneville, P. L.; Macartney, J. H. The behavior of aliphatic aldehydes in the Leuckart-Wallach reaction. J. Am. Chem. Soc. 1950, 72, 3073–3075. [Google Scholar] [CrossRef]
- Rivera, A.; Ríos-Motta, J. An unusual product obtained from condensation between ethylenediamine and formaldehyde in basic medium. Tetrahedron Lett. 2005, 46, 5001–5004. [Google Scholar] [CrossRef]
- Lambert, J. B.; Majchrzak, M. W. Ring-chain tautomerism in 1,3-dimethylimidazolidine on the NMR time scale. J. Am. Chem. Soc. 1979, 101, 1048–1049. [Google Scholar] Lambert, J. B.; Majchrzak, M. W. Ring-chain tautomerism in 1,3-diaza and 1,3-oxaza heterocycles. J. Am. Chem. Soc. 1980, 102, 3588–3591. [Google Scholar] [CrossRef]
- Pliego, J. R.; Alcântara, A. F. C.; Veloso, D. P.; de Almeida, W. B. Theoretical and experimental investigation of the formation of E and Z-aldimines from the reaction of methylamine with acetaldehyde. J. Braz. Chem. Soc. 1999, 10, 381–388. [Google Scholar]
- Eldin, S.; Digits, J. A.; Huang, S.-T.; Jencks, W. P. Lifetime of an aliphatic iminium ion in aqueous solution. J. Am. Chem. Soc. 1995, 117, 6631–6632. [Google Scholar] Eldin, S.; Jencks, W. P. Lifetimes of iminium ions in aqueous solution. J. Am. Chem. Soc. 1995, 117, 4851–4857. [Google Scholar]
- Frisch, M. J.; Trucks, G. W.; Robb, M. A.; Schlegel, H. B.; Scuseria, G. E.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A., Jr.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T. M.; Al-Laham, A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M. P.; Gill, M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 98, Revision A.9; Gaussian, Inc.: Pittsburgh, PA, 1998. [Google Scholar]
- Peori, M. B.; Vaughan, K.; Hooper, D. L. Synthesis and characterization of novel bis-triazenes:3,8-di[2-aryl-1-azenyl]-1,3,6,8-tetraazabicyclo[4.4.1]undecanes and 1,3-di-2-[(4-methoxyphenyl)-1-diazenyl]imidazolidine. The reaction of diazonium ions with ethylenediamine/formaldehyde mixtures. J. Org. Chem. 1998, 63, 7437–7444. [Google Scholar] [CrossRef]
- Simkins, R. J. J.; Wright, G. F. Nitrolysis of 1,3-6,8-Diendomethylene-l,3,6,8-tetrazacyclodecane. J. Am. Chem. Soc. 1955, 77, 3157–3159. [Google Scholar]
- Sample Availability: Samples of compound 6 are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Rivera, A.; Rios-Motta, J. Unusual Reactivity Patterns of 1,3,6,8-Tetraazatricyclo-[4.4.1.13,8]-dodecane (TATD) Towards Some Reducing Agents: Synthesis of TMEDA. Molecules 2007, 12, 1471-1481. https://doi.org/10.3390/12071471
Rivera A, Rios-Motta J. Unusual Reactivity Patterns of 1,3,6,8-Tetraazatricyclo-[4.4.1.13,8]-dodecane (TATD) Towards Some Reducing Agents: Synthesis of TMEDA. Molecules. 2007; 12(7):1471-1481. https://doi.org/10.3390/12071471
Chicago/Turabian StyleRivera, Augusto, and Jaime Rios-Motta. 2007. "Unusual Reactivity Patterns of 1,3,6,8-Tetraazatricyclo-[4.4.1.13,8]-dodecane (TATD) Towards Some Reducing Agents: Synthesis of TMEDA" Molecules 12, no. 7: 1471-1481. https://doi.org/10.3390/12071471



