Substituted N-Phenylpyrazine-2-carboxamides, Their Synthesis and Evaluation as Herbicides and Abiotic Elicitors
Abstract
:Introduction
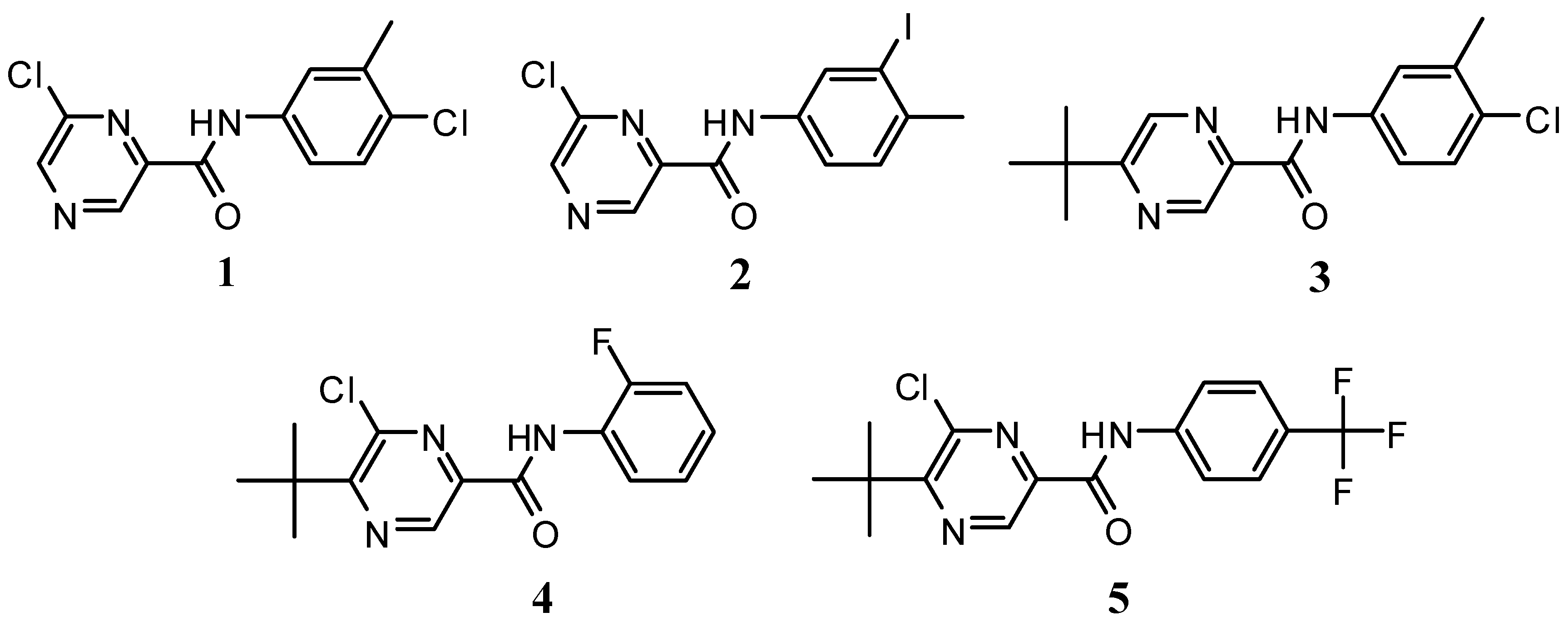
Results and Discussion
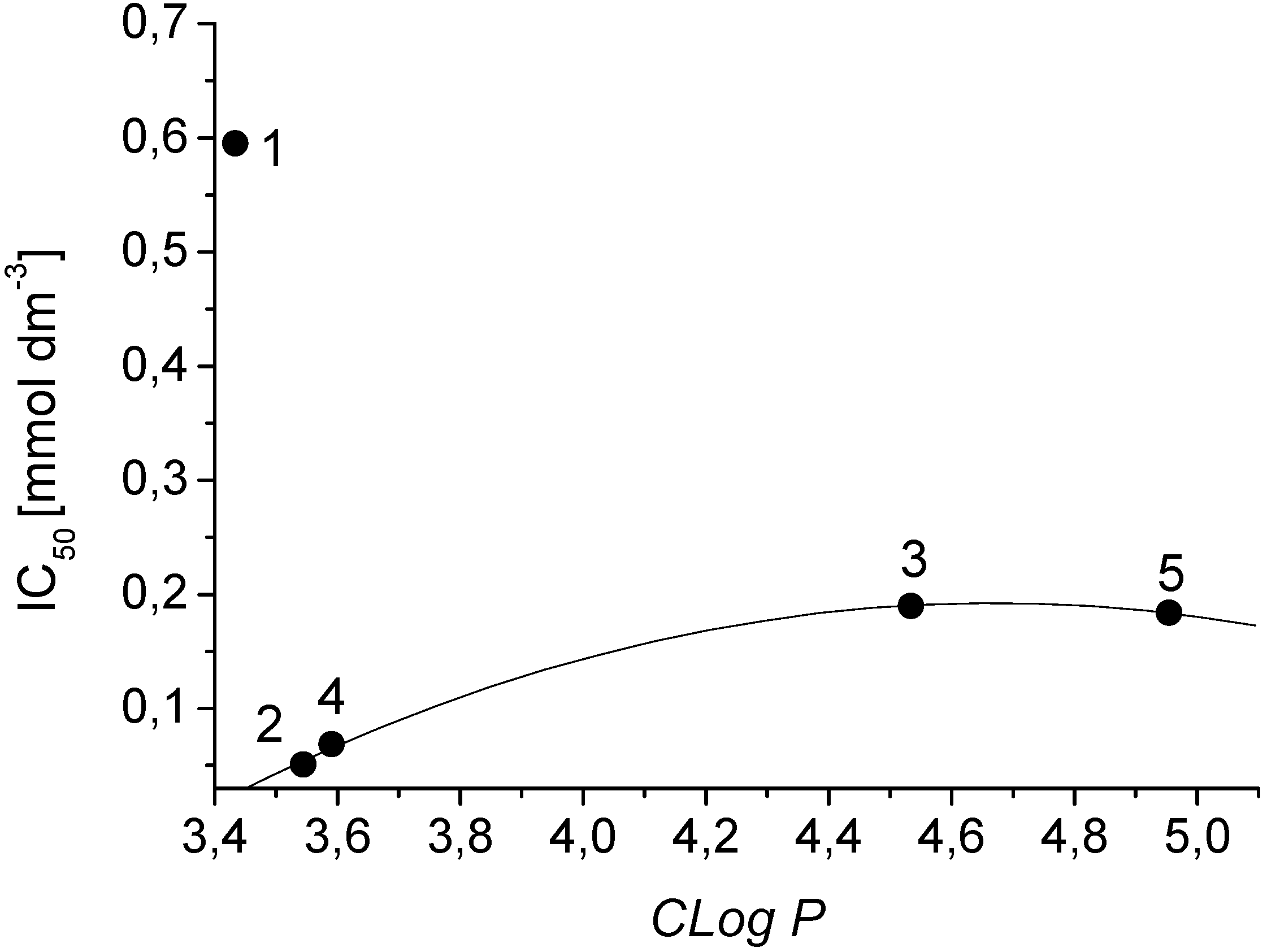
| Comp. | OER inhibition Spinacia oleracea IC50 [mmol/L] | Chlorophyll reduction Chlorella vulgaris IC50 [mmol/L] | Maximal flavonoid content (%) after elicitation/hours | Flavonoid content (%) without elicitation | Lipophilicity Log P / CLog P |
| 1 | 0.595 | 0.080 | 0.0987 / 6 | 0.0557 | 2.53 / 3.43369 |
| 2 | 0.051 | a | 0.5072 / 12 | 0.0557 | 3.33 / 3.54369 |
| 3 | 0.190 | 0.044 | 0.1007 / 48 | 0.0557 | 3.76 / 4.53404 |
| 4 | 0.069 | 0.089 | 0.0759 / 6 | 0.0557 | 3.78 / 3.59069 |
| 5 | 0.184 | a | 0.1839 / 168 | 0.0557 | 4.54 / 4.95436 |
| DCMU | 0.0019 | 0.0073 | - | - | 2.76 / 2.69124 |

Experimental
General
General procedure for pyrazinecarboxamide synthesis.
Lipophilicity calculations
Herbicidal activities
Study of the inhibition of oxygen evolution rate in spinach chloroplasts.
Reduction of chlorophyll content in the green algaeChlorella vulgaris Beij.
Abiotic elicitacion
Study of the substituted pyrazinecarboxylic acids on flavonoid production in vitro cultures.
Evaluation of flavonoid Stock solution [23].
Test solution.
Compensation solution.
Acknowledgments
References
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Eq. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Dolezal, M. Biological active pyrazines of natural and synthetic origin. Chem. Listy 2006, 100, 959–966. [Google Scholar]
- Dolezal, M.; Palek, L.; Vinsova, J.; Buchta, V.; Jampilek, J.; Kralova, K. Substituted Pyrazinecarboxamides; Synthesis and Their Biological Evaluation. Molecules 2006, 11, 242–256. [Google Scholar] [CrossRef]
- Dolezal, M.; Cmedlova, P.; Palek, L.; Vinsova, J.; Kunes, J.; Buchta, V.; Jampilek, J.; Kralova, K. Synthesis and antimycobacterial evaluation of substituted pyrazinecarboxamides. Eur. J. Med. Chem. 2008, 43. in press. [Google Scholar]
- Janin, Y. L. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007, 15, 2479–2513. [Google Scholar] [CrossRef]
- Tripathi, R. P.; Tewari, N.; Dwivedi, N.; Tiwari, V. K. Fighting tuberculosis: An old disease with new challenges. Med. Res. Rev. 2005, 25, 93–131. [Google Scholar] [CrossRef]
- Ricci, D.; Maggiali, C.A.; Ronchini, F.; Tirillini, B.; Fraternale, D. Phytochemistry 1991, 30, 2821–2824. [CrossRef]
- Camper, N. D.; McDonald, S. K. Tissue and cell cultures as model system in herbicide research. Rev. Weed Sci. 1989, 4, 169–190. [Google Scholar]
- Linsmaier, E.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Schmitz, R. V.; Skoog, F.; Hecht, S. M.; Leonard, N. J. Comparison of zeatin indoleacetate with zeatin and indoleacetic acid in tobacco bioassay. Plant. Physiol. 1972, 50, 114–116. [Google Scholar] [CrossRef]
- Huffman, J. B.; Camper, N. D. Growth inhibition in tobacco (Nicotiana tabacum) callus by 2,6-dinitroaniline herbicides and protection by D-α-tocopherol acetate. Weed Sci. 1978, 26, 527–530. [Google Scholar]
- Tumova, L.; Ostrozlik, P. Ononis arvensis in vitro - Abiotic elicitation. Cesk. Slov. Farm. 2002, 51, 173–176. [Google Scholar]
- Tumova, L.; Gallova, K.; Rimakova, J.; Dolezal, M.; Tuma, J. The effect of substituted amides of pyrazine-2-carboxylic acids on flavolignan production in Silybum marianum culture in vitro. Acta Physiol. Plant. 2005, 27, 357–362. [Google Scholar]
- Dolezal, M.; Kralova, K.; Sersen, F.; Miletin, M. The site of action of some anilides of pyrazine-2-carboxylic acids in the photosynthetic apparatus. Folia Pharm. Univ. Carol. 2001, 26, 13–20. [Google Scholar]
- Abe, Y.; Shigeta, Y.; Uchimaru, F.; Okada, S.; Ozasayama, E. Japanese Patent 69 12,898, 1969. [Chem. Abstr. 1969, 71, 112979y].
- Dolezal, M.; Hartl, J.; Miletin, M.; Machacek, M.; Kralova, K. Chem. Pap. 1999, 53, 126–128.
- Walker, D. A. Methods in Enzymology Part C; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, 1980; Vol. 69, pp. 94–104. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- Fedke, C. Biochemistry and Physiology of Herbicide Action; Springer Verlag: Berlin-Heidelberg-New York, 1982. [Google Scholar]
- Kralova, K.; Sersen, F.; Melnik, M. Inhibition of photosynthesis in Chlorella vulgaris by Cu(II) complexes with biologically active ligands. J. Trace Microprobe Techn. 1998, 16, 491–500. [Google Scholar]
- Wellburn, A. R. The spectral determination of chlorophyll-A and chlorophyll-B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Czech Pharmacopea 1997; Grada: Prague, 1997; p. 1491.
- Sample Availability: Samples of the compounds 1-5 are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Doležal, M.; Tůmová, L.; Kešetovičová, D.; Tůma, J.; Kráľová, K. Substituted N-Phenylpyrazine-2-carboxamides, Their Synthesis and Evaluation as Herbicides and Abiotic Elicitors. Molecules 2007, 12, 2589-2598. https://doi.org/10.3390/12122589
Doležal M, Tůmová L, Kešetovičová D, Tůma J, Kráľová K. Substituted N-Phenylpyrazine-2-carboxamides, Their Synthesis and Evaluation as Herbicides and Abiotic Elicitors. Molecules. 2007; 12(12):2589-2598. https://doi.org/10.3390/12122589
Chicago/Turabian StyleDoležal, Martin, Lenka Tůmová, Diana Kešetovičová, Jiří Tůma, and Katarína Kráľová. 2007. "Substituted N-Phenylpyrazine-2-carboxamides, Their Synthesis and Evaluation as Herbicides and Abiotic Elicitors" Molecules 12, no. 12: 2589-2598. https://doi.org/10.3390/12122589




